AP Chemistry
DrOfEng
- 18 / 21
1
Introduction to Chemistry, Periodic Table - AP Chemistry
DrOfEng
This video provides an introduction to chemistry, and covers some important features of the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
2
Atoms, Isotopes, Atomic Number, Mass Number - AP Chemistry
DrOfEng
This AP Chemistry video explains the notation used in the periodic table, and the structure of atoms and isotopes.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
3
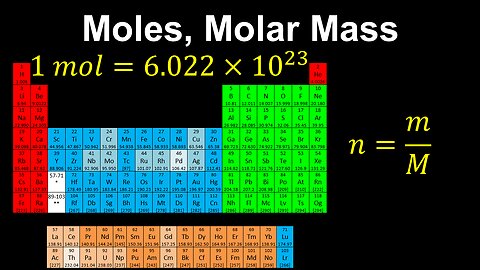
Moles, Molar Mass, Avogadro's Number - AP Chemistry
DrOfEng
This AP Chemistry video defines the unit of mole using Avogadro's number, which is used to connect the mass of a substance to the number of its particles.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
4
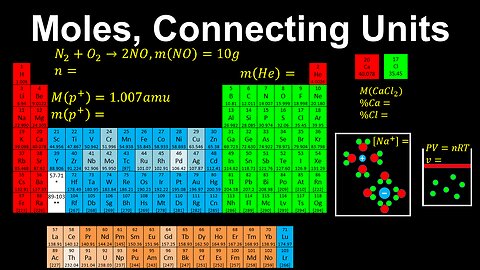
Moles, Connecting Units - AP Chemistry
DrOfEng
This AP Chemistry video explains how the unit of mole connects other units in Chemistry.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
5
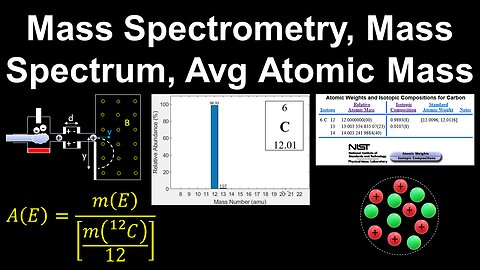
Mass Spectrometry, Molar Mass, Average Atomic Mass, Mass Defect - AP Chemistry
DrOfEng
This AP Chemistry video explains mass spectrometry and how it is used to identify the isotopes of an element, interpreting the mass spectrum, relationship between the molar mass and average atomic mass, and the mass defect.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
6
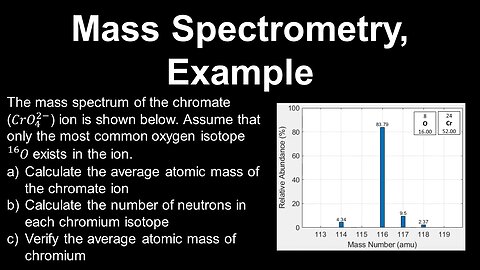
Mass Spectrometry, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on mass spectrometry.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
7
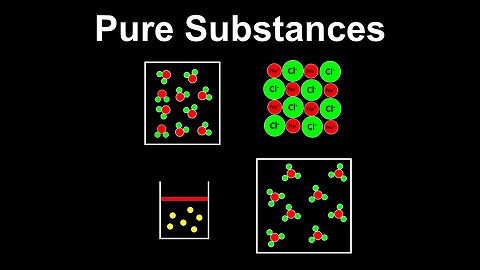
Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video defines pure substances using several examples.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
8
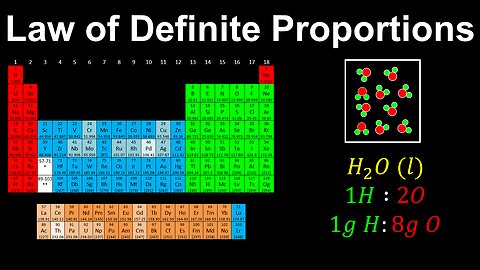
Law of Definite Proportions, Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video explains the law of definite proportions for pure substances.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
9
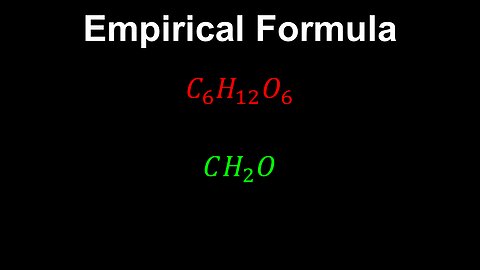
Empirical Formula, Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video defines the empirical formula for pure substances.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
10
Pure Substances, Percent Composition, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on determining the percentage composition of each element in a pure substance.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
11
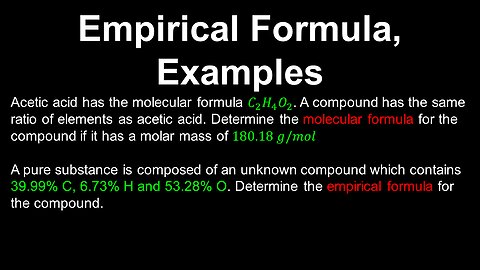
Empirical Formula, Molecular Formula, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on finding the empirical or molecular formula of a compound in a pure substance.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
12
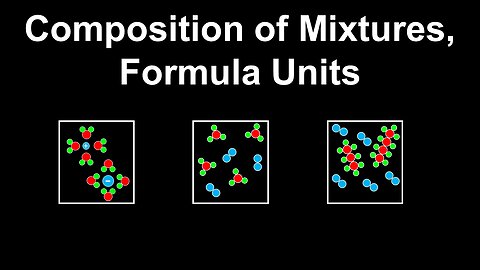
Composition of Mixtures, Formula Units - AP Chemistry
DrOfEng
This AP Chemistry video explains the composition of mixtures and formula units using several examples.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
13
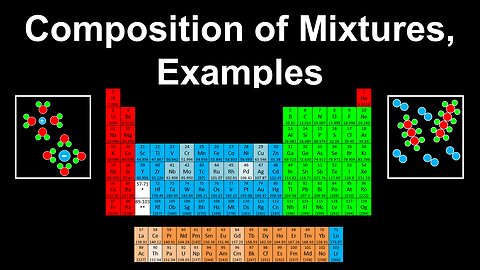
Composition of Mixtures, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on the composition of mixtures.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
14
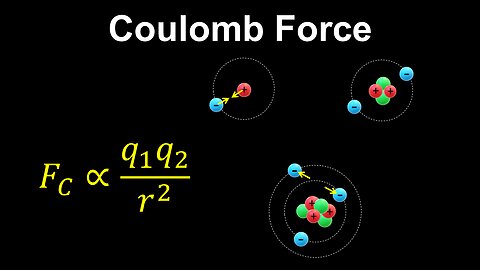
Coulomb Force, Bohr Model, Atoms - AP Chemistry
DrOfEng
This AP Chemistry video explains Coulomb's law and the forces between charges in an atom using the simplified Bohr model.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
15
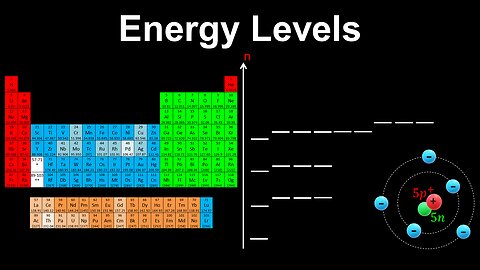
Energy Levels, Shells, Subshells, Orbitals - AP Chemistry
DrOfEng
This AP Chemistry video explains the types of shells, subshells and orbitals present in an atom at different energy levels.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
16
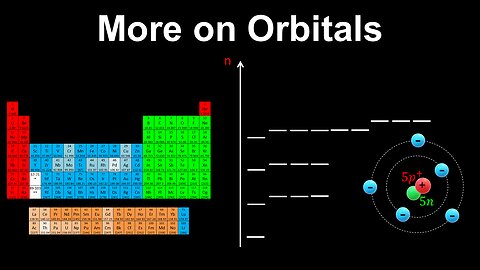
Shapes of Orbitals - AP Chemistry
DrOfEng
This AP Chemistry video explains the shapes of s and p orbitals, which are obtained from quantum mechanics.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
17
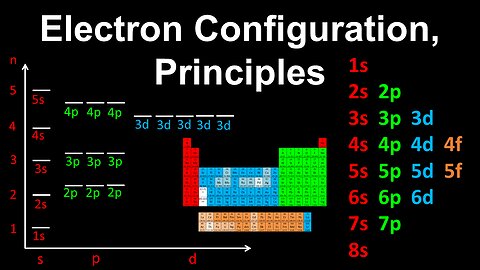
Electron Configuration, Aufbau, Pauli's Exclusion, Hund's Rule - AP Chemistry
DrOfEng
This AP Chemistry video explains Aufbau's rule, Pauli's exclusion principle and Hund's rule for determining the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
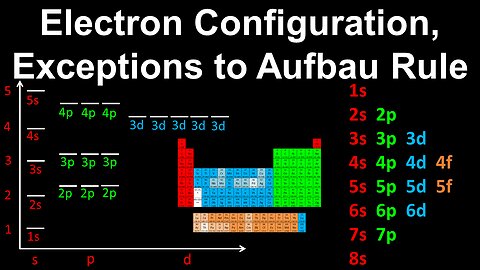
Electron Configuration, Exceptions to the Aufbau Rule, Chromium - AP Chemistry
DrOfEng
This AP Chemistry video explains how to determine the electron configuration for an atom which is an exception to Aufbau's rule, using Chromium as an example.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
19
Electron Configuration, Silicon, Iron, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on determining the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
20
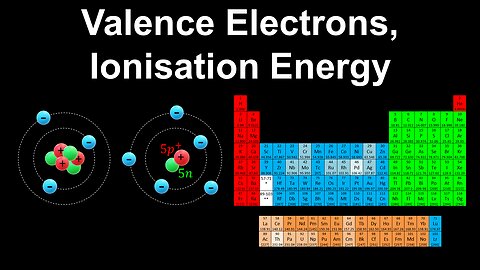
Valence Electrons, Noble Gas Notation, Ionisation Energy - AP Chemistry
DrOfEng
This AP Chemistry video defines valence electrons, explains how to write the electron configuration of an atom using shorthand (or noble gas) notation, and how to qualitatively estimate the ionisation energy using the energy analog of Coulomb's law.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
21
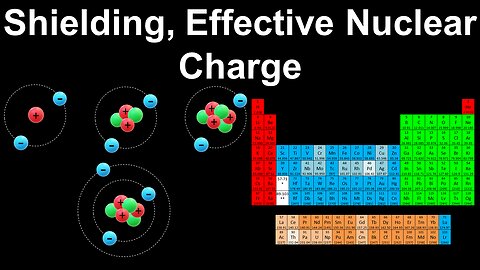
Shielding, Effective Nuclear Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains the concepts of electron shielding and effective nuclear charge to determine the ionisation energy of atoms and ions relative to each other.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
Electron Configuration, Exceptions to the Aufbau Rule, Chromium - AP Chemistry
Enjoyed this video? Join my Locals community for exclusive content at
drofeng.locals.com!
17 days ago
2
This AP Chemistry video explains how to determine the electron configuration for an atom which is an exception to Aufbau's rule, using Chromium as an example.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
Loading comments...
-
 9:31
9:31
ChemistryFiles
3 months agoElectron Configuration
4 -
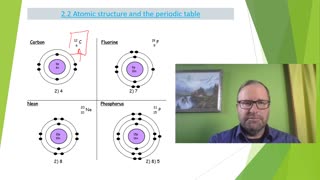 12:29
12:29
AchieveYourPotential
1 year agoAtomic Structure and the Periodic Table, Electron Configuration Part 3
5 -
 25:57
25:57
Chemistry Tutor
4 years agoElectron Arrangement & Octet Rule Video Chemistry for Nurses (Lecture 9)
10 -
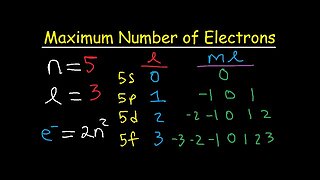 11:45
11:45
TheOrganicChemistryTutor
10 months agoHow To Determine The Maximum Number of Electrons Using Allowed Quantum Numbers - 8 Cases
193 -
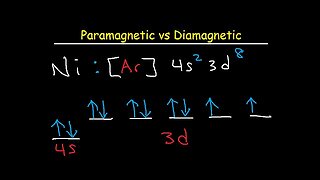 11:01
11:01
TheOrganicChemistryTutor
7 months agoParamagnetic vs Diamagnetic - Paired vs Unpaired Electrons - Electron Configuration
93 -
 4:24
4:24
TheOrganicChemistryTutor
10 months agoHow To Determine The 4 Quantum Numbers From an Element or a Valence Electron
18 -
 3:24
3:24
Dr. Lodi
24 days agoWhat Causes Electrons To Move?
1811 -
 2:39
2:39
Chemistry Tutor
1 year agoStereochemistry - Using CIP Rules to Assign Priority to Atoms Help Me With Organic Chemistry!
56 -
 2:31
2:31
Chemistry Tutor
1 year agoOrganic Chemistry Orbital Overlap Drawing of Methane (CH4) sp3 hybrid orbitals
7 -
 14:26
14:26
DTM High School Chemistry
1 year agoUnit 3 The Atom Recording 4 Valence Electrons
2