AP Chemistry
DrOfEng
- 10 / 75
1
Introduction to Chemistry, Periodic Table - AP Chemistry
DrOfEng
This video provides an introduction to chemistry, and covers some important features of the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
2
Atoms, Isotopes, Atomic Number, Mass Number - AP Chemistry
DrOfEng
This AP Chemistry video explains the notation used in the periodic table, and the structure of atoms and isotopes.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
3
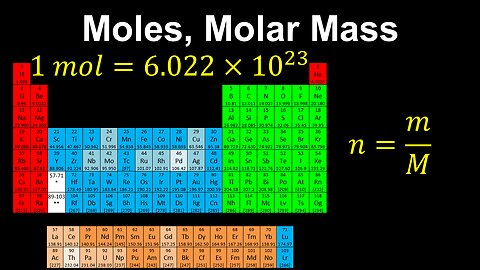
Moles, Molar Mass, Avogadro's Number - AP Chemistry
DrOfEng
This AP Chemistry video defines the unit of mole using Avogadro's number, which is used to connect the mass of a substance to the number of its particles.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
4
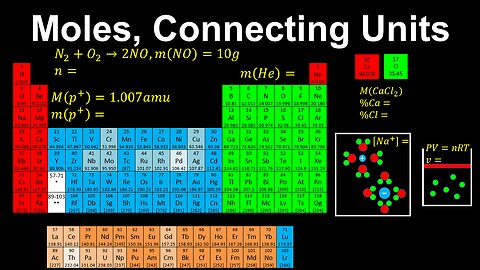
Moles, Connecting Units - AP Chemistry
DrOfEng
This AP Chemistry video explains how the unit of mole connects other units in Chemistry.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
5
Mass Spectrometry, Molar Mass, Average Atomic Mass, Mass Defect - AP Chemistry
DrOfEng
This AP Chemistry video explains mass spectrometry and how it is used to identify the isotopes of an element, interpreting the mass spectrum, relationship between the molar mass and average atomic mass, and the mass defect.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
6
Mass Spectrometry, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on mass spectrometry.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
7
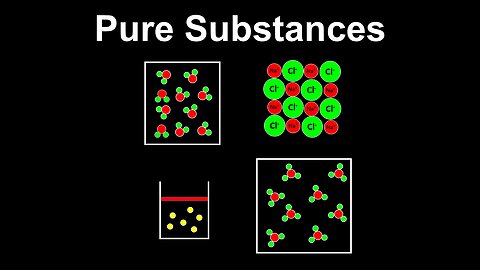
Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video defines pure substances using several examples.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
8
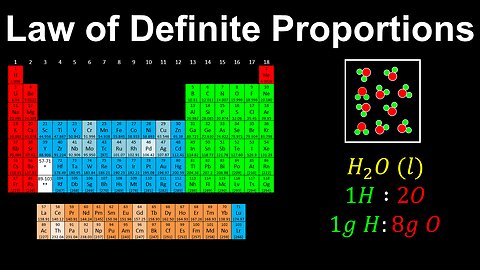
Law of Definite Proportions, Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video explains the law of definite proportions for pure substances.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
9
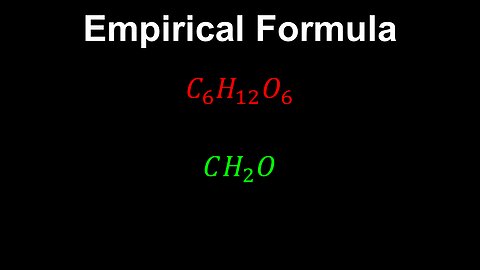
Empirical Formula, Pure Substances - AP Chemistry
DrOfEng
This AP Chemistry video defines the empirical formula for pure substances.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
Pure Substances, Percent Composition, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on determining the percentage composition of each element in a pure substance.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
11
Empirical Formula, Molecular Formula, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on finding the empirical or molecular formula of a compound in a pure substance.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
12
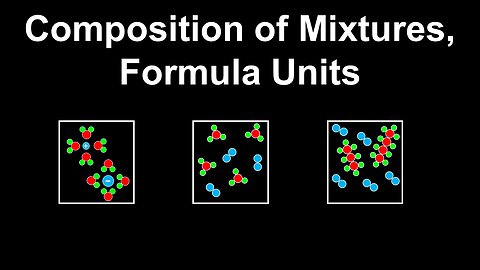
Composition of Mixtures, Formula Units - AP Chemistry
DrOfEng
This AP Chemistry video explains the composition of mixtures and formula units using several examples.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
13
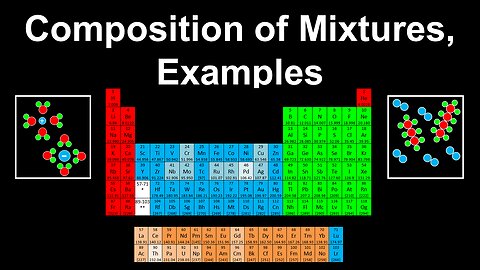
Composition of Mixtures, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on the composition of mixtures.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
14
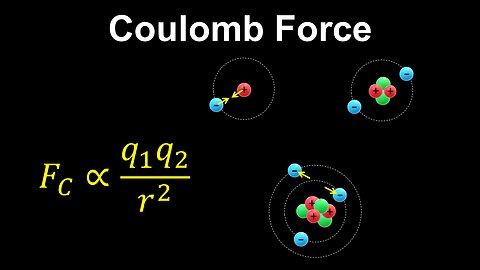
Coulomb Force, Bohr Model, Atoms - AP Chemistry
DrOfEng
This AP Chemistry video explains Coulomb's law and the forces between charges in an atom using the simplified Bohr model.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
15
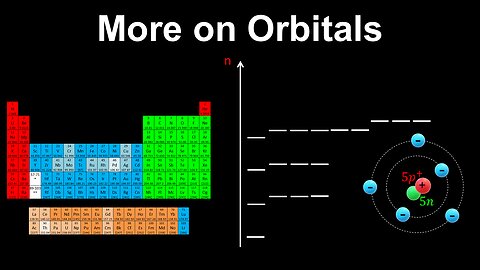
Energy Levels, Shells, Subshells, Orbitals - AP Chemistry
DrOfEng
This AP Chemistry video explains the types of shells, subshells and orbitals present in an atom at different energy levels.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
16
Shapes of Orbitals - AP Chemistry
DrOfEng
This AP Chemistry video explains the shapes of s and p orbitals, which are obtained from quantum mechanics.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
17
Electron Configuration, Aufbau, Pauli's Exclusion, Hund's Rule - AP Chemistry
DrOfEng
This AP Chemistry video explains Aufbau's rule, Pauli's exclusion principle and Hund's rule for determining the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
18
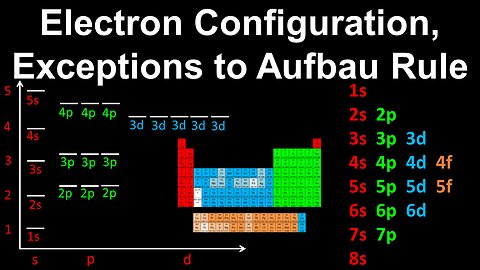
Electron Configuration, Exceptions to the Aufbau Rule, Chromium - AP Chemistry
DrOfEng
This AP Chemistry video explains how to determine the electron configuration for an atom which is an exception to Aufbau's rule, using Chromium as an example.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
19
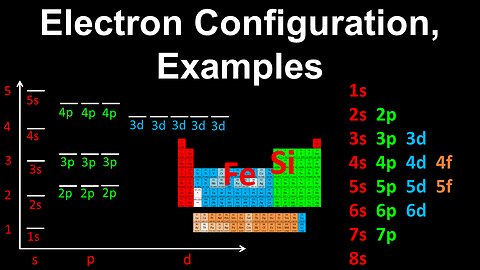
Electron Configuration, Silicon, Iron, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on determining the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
20
Valence Electrons, Noble Gas Notation, Ionisation Energy - AP Chemistry
DrOfEng
This AP Chemistry video defines valence electrons, explains how to write the electron configuration of an atom using shorthand (or noble gas) notation, and how to qualitatively estimate the ionisation energy using the energy analog of Coulomb's law.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
21
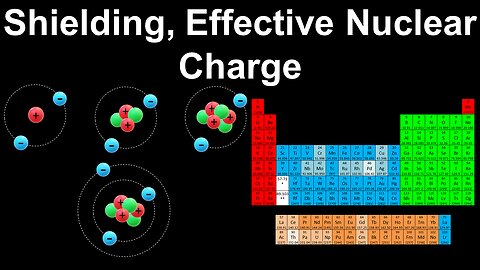
Shielding, Effective Nuclear Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains the concepts of electron shielding and effective nuclear charge to determine the ionisation energy of atoms and ions relative to each other.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
22
Photoelectron Spectroscopy - AP Chemistry
DrOfEng
This AP Chemistry video explains the steps of photoelectron spectroscopy, which is an experiment used to determine the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
23
Photoelectron Spectrum, Interpretation - AP Chemistry
DrOfEng
This AP Chemistry video explains how to interpret a photoelectron spectrum to determine the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
24
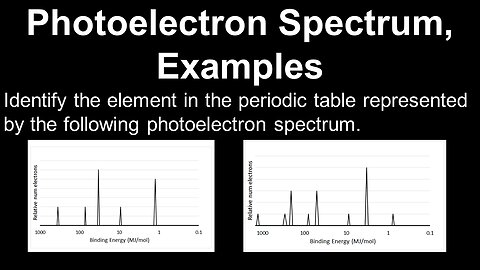
Photoelectron Spectrum, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on interpreting a photoelectron spectrum to determine the electron configuration of an atom.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
25
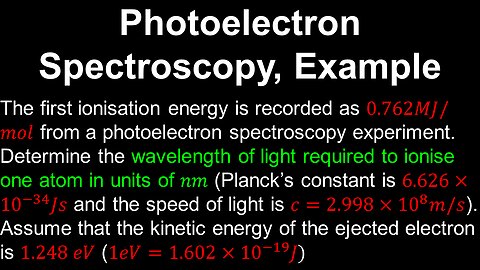
Photoelectron Spectroscopy, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on obtaining the parameters of the electromagnetic radiation source from a photoelectron spectroscopy experiment.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
26
Periodic Trends, Ionisation Energy - AP Chemistry
DrOfEng
This AP Chemistry video explains the periodic trend in ionisation energy using the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
27
Periodic Trends, Atomic Radius - AP Chemistry
DrOfEng
This AP Chemistry video explains the periodic trend in atomic radius using the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
28
Periodic Trends, Ionic Radius, Cation, Anion - AP Chemistry
DrOfEng
This AP Chemistry video explains the periodic trend in ionic radius using the periodic table, and compares the atomic radii and ionic radii of atoms and ions.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
29
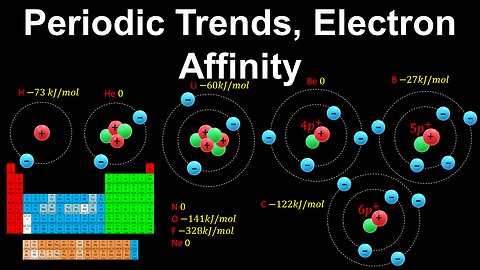
Periodic Trends, Electron Affinity - AP Chemistry
DrOfEng
This AP Chemistry video explains the periodic trend in electron affinity using the periodic table, which is the energy released when an electron is attached to an atom to form an anion.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
30
Periodic Table, Trends, Electronegativity - AP Chemistry
DrOfEng
This AP Chemistry video explains the periodic trend in electronegativity using the periodic table, which is the ability of an atom to attract an electron from another atom to form a chemical bond.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
31
Periodic Table, Trends, Worked Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on periodic trends in the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
32
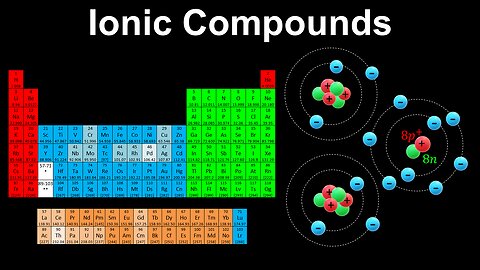
Ionic Compounds - AP Chemistry
DrOfEng
This AP Chemistry video explains how ionic compounds are formed and the number of charges gained or lost based on the groups of the elements in the periodic table.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
33
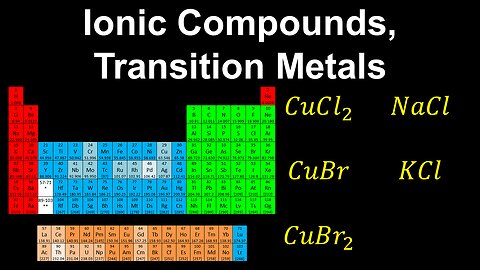
Ionic Compounds from Transition Metals, Analogous Compounds - AP Chemistry
DrOfEng
This AP Chemistry video explains how to name different ionic compounds formed from a transition metal, and analogous ionic compounds.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
34
Chemical Bond Types, Electronegativity Pauling Scale - AP Chemistry
DrOfEng
This AP Chemistry video summarises the types of chemical bonds, being ionic, covalent (polar and non-polar) and metallic.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
35
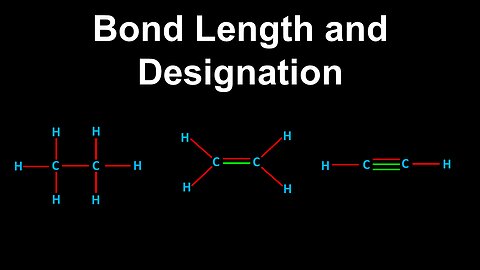
Covalent Bond Designations, Bond Length - AP Chemistry
DrOfEng
This AP Chemistry video explains the properties of single, double and triple covalent bonds.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
36
Covalent Bonds, Polarity, Dipole Moment - AP Chemistry
DrOfEng
This AP Chemistry video explains the polarity of covalent bonds, dipole moments and how to determine whether a bond is covalent or ionic.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
37
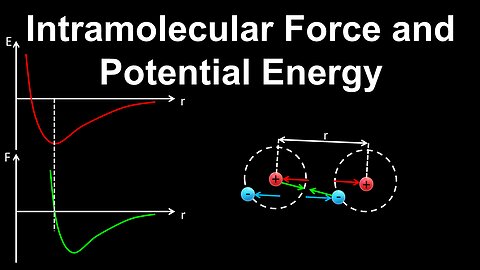
Intramolecular Force, Potential Energy, Covalent Bonds - AP Chemistry
DrOfEng
This AP Chemistry video explains how to interpret the potential energy diagram for a covalent bond, and identify the bond length and bond energy.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
38
Interactions, Cations and Anions, Coulomb's Law - AP Chemistry
DrOfEng
This AP Chemistry video explains the interactions between cations and anions using Couloumb's law.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
39
Ionic Solids, Structure, Properties - AP Chemistry
DrOfEng
This AP Chemistry video explains the structure and properties of ionic solids.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
40
Structure of Metals, Sea of Electrons Model - AP Chemistry
DrOfEng
This AP Chemistry video explains the structure and properties of metals, and metallic bonds using the sea of electrons model.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
41
Interstitial Alloys, Metals - AP Chemistry
DrOfEng
This AP Chemistry video explains the structure of interstitial alloys using steel as a typical example.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
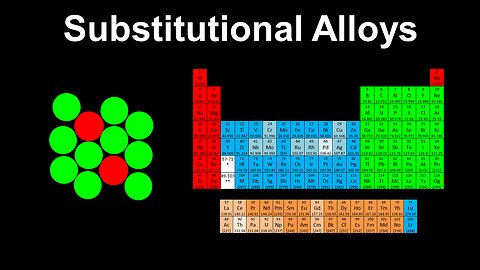
42
Substitutional Alloys, Metals - AP Chemistry
DrOfEng
This AP Chemistry video explains the structure of substitutional alloys using brass as a typical example.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
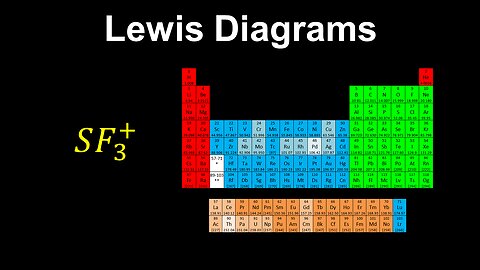
43
Lewis Diagrams - AP Chemistry
DrOfEng
This AP Chemistry video explains how to draw a Lewis diagram for a molecule or polyatomic ion using a set of rules.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
44
Lewis Diagrams, Resonance Structures, Bond Order - AP Chemistry
DrOfEng
This AP Chemistry video explains how to draw Lewis diagrams of the resonance structures of a molecule or polyatomic ion and determine the bond order.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
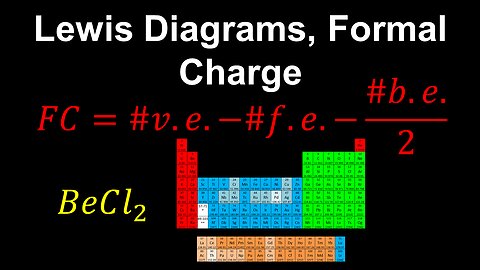
45
Lewis Diagrams, Formal Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains how the formal charge can be calculated and used to determine the most likely correct Lewis diagram of a molecule or polyatomic ion.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
46
Lewis Diagrams, Reduced Octet, Formal Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains how to draw the Lewis diagram for a molecule or polyatomic ion where the central atom has a reduced octet.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
47
Lewis Diagrams, Expanded Octet , Formal Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains how to draw the Lewis diagram for a molecule or polyatomic ion where the central atom has an expanded octet.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
48
Lewis Diagrams, Free Radical, Formal Charge - AP Chemistry
DrOfEng
This AP Chemistry video explains how to draw the Lewis diagram for a free radical (has unpaired electrons).
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
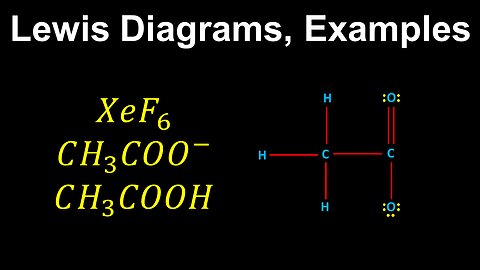
49
Lewis Diagrams, Resonance, Formal Charge, Worked Examples, XeF6, CH3COO-, CH3COOH - AP Chemistry
DrOfEng
This AP Chemistry video covers worked examples on Lewis diagrams.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
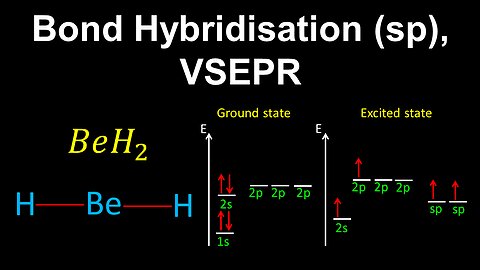
50
sp Hybridisation, VSEPR, BeH2 - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp hybridised.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
51
sp2 Hybridisation, Trigonal Planar, VSEPR - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp2 hybridised with 3 sigma bonds.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
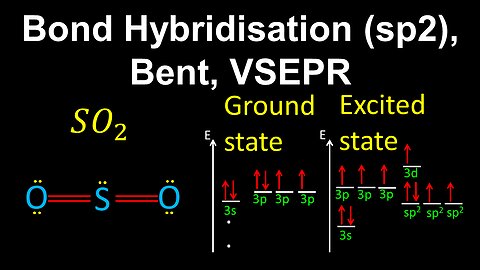
52
sp2 Hybridisation, Bent, VSEPR - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp2 hybridised with 2 sigma bonds and a lone pair of electrons.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
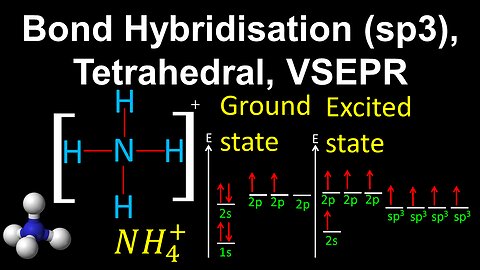
53
sp3 Hybridisation, Tetrahedral, VSEPR - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp3 hybridised with 4 sigma bonds.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
54
sp3 Hybridisation, Trigonal Pyramidal, VSEPR - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp3 hybridised with 3 sigma bonds and a lone pair of electrons.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
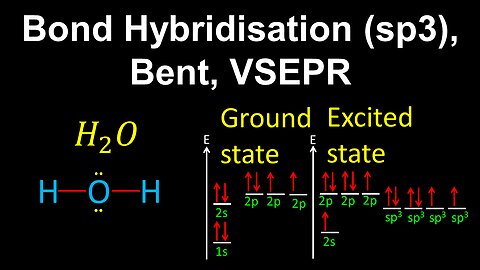
55
sp3 Hybridisation, Bent, VSEPR - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw the geometry of a molecule using bond hybridisation and VSEPR, where the central atom is sp3 hybridised with 2 sigma bonds and two lone pairs of electrons.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
56
Steric Number, VSEPR, Molecular Geometry - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to quickly determine the geometry of a molecule or polyatomic ion using the steric number.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
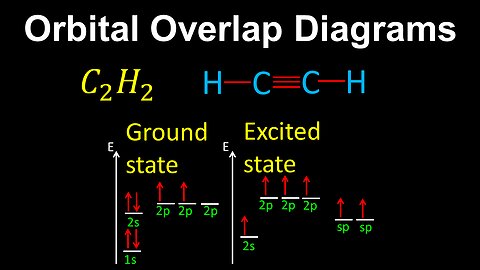
57
Orbital Overlap Diagrams, Bond Formation - AP Chemistry
DrOfEng
This AP Chemistry video demonstrates how to draw orbital overlap diagrams, where the overlap between electron orbitals results in sigma and pi bonds.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
58
Structural Isomers, pi Bonds - AP Chemistry
DrOfEng
This AP Chemistry video covers structural isomers with pi bonds and explains how to determine the geometry of a large molecule.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
59
Bond Hybridisation, Molecular Geometry, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on bond hybridisation and molecular geometry.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
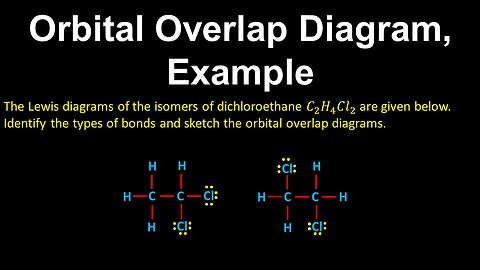
60
Orbital Overlap Diagram, Bonds, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on orbital overlap diagrams.
This video is part of Unit 2 of AP Chemistry on Molecular and Ionic Compound Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
61
Polarity of Molecules - AP Chemistry
DrOfEng
This AP Chemistry video explains how to determine the polarity of a molecular using the polarity of the covalent bonds and symmetry in the molecule's geometry.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
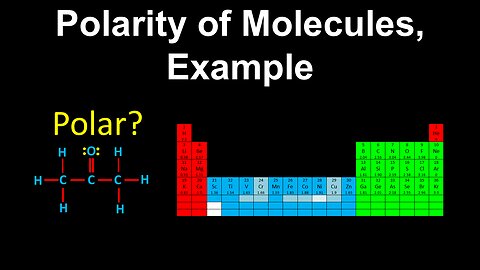
62
Polarity of Molecules, Example - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on the polarity of molecules.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
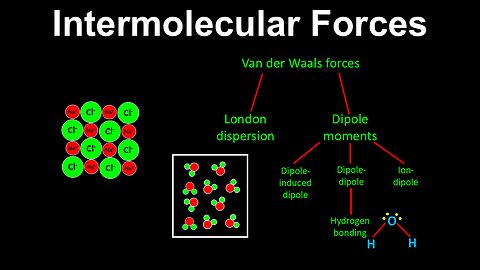
63
Intermolecular Forces, van der Waals forces, Introduction - AP Chemistry
DrOfEng
This AP Chemistry video provides an introduction to intermolecular forces.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
64
London Dispersion Forces, Temporary Fluctuating Dipoles - AP Chemistry
DrOfEng
This AP Chemistry video explains London Dispersion Forces, which are weak intermolecular interactions that are most prominent in large molecules, and can also occur between non-polar molecules.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
65
Dipole-Induced Dipole Interactions - AP Chemistry
DrOfEng
This AP Chemistry video explains dipole-induced dipole interactions which are forces between polar and non-polar molecules.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
66
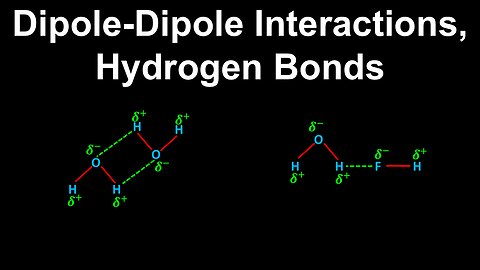
Dipole-Dipole Interactions, Hydrogen Bonds - AP Chemistry
DrOfEng
This AP Chemistry video explains dipole-dipole interactions which are forces between polar molecules, and hydrogen bonding, which is a strong type of dipole-dipole interaction between polar molecules.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
67
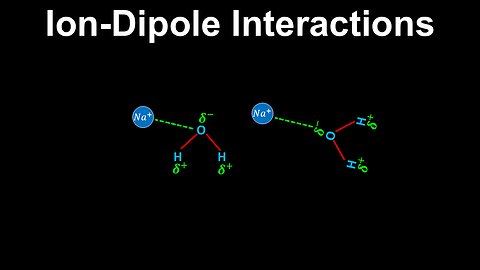
Ion-Dipole Interactions - AP Chemistry
DrOfEng
This AP Chemistry video explains ion-dipole interactions, which is a strong interaction between an ion and polar molecule.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
68
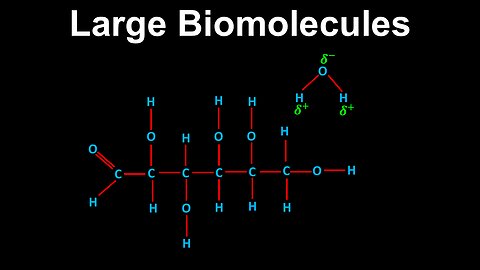
Large Biomolecules, Non-Covalent Interactions - AP Chemistry
DrOfEng
This AP Chemistry video explains non-covalent interactions in large biomolecules, which can occur within the same biomolecule and with other molecules.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
69
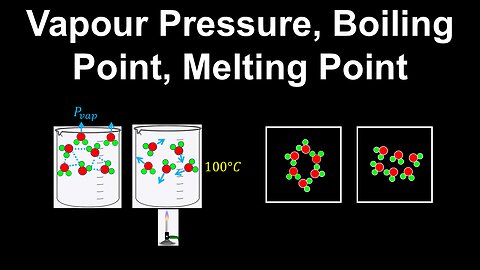
Vapour Pressure, Boiling Point, Melting Point - AP Chemistry
DrOfEng
This AP Chemistry video explains the main properties of solids and liquids (vapour pressure, boiling point and melting point) and how they are related to the intermolecular forces.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
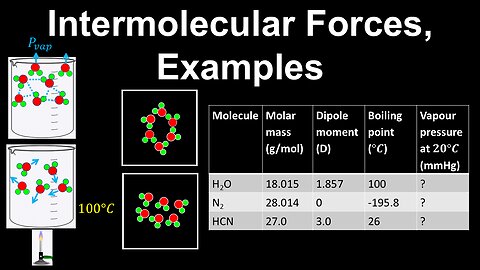
70
Intermolecular Forces, Vapour Pressure, Boiling Point, Examples - AP Chemistry
DrOfEng
This AP Chemistry video covers a worked example on intermolecular forces, vapour pressure and boiling point.
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
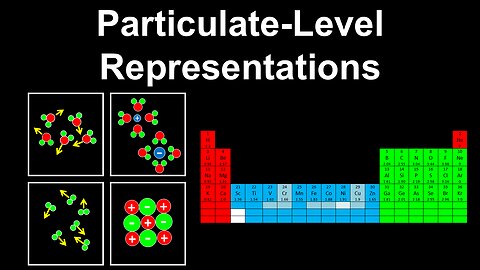
71
Particulate-Level Representations, Chemical Species, Interactions - AP Chemistry
DrOfEng
This AP Chemistry video explains how particulate-level representations can be used to visualise different chemical species and the interactions between them, which leads to establishing their macroscopic properties (e.g. vapour pressure, boiling point, melting point).
This video is part of Unit 3 of AP Chemistry on Intermolecular Forces and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
1
comment
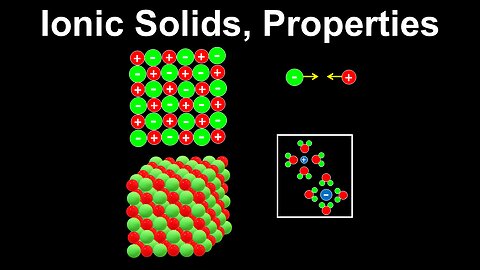
72
Ionic Solids - Chemistry
DrOfEng
This chemistry video covers a tutorial on ionic solids. It explains how the lattice structure of ionic solids and the Coulomb interactions between the ions are used to describe their properties including low vapour pressure, high boiling and melting points, brittleness and poor conductivity in the solid phase. The cations and anions in ionic solids are arranged to minimise repulsion between opposite charges. In the liquid state, the ions are mobile and conduct electricity.
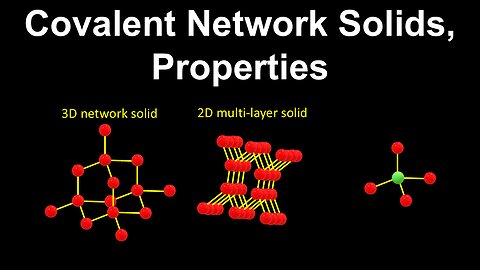
73
Covalent Network Solids - Chemistry
DrOfEng
This chemistry video covers a tutorial on covalent network solids. It explains how the chemical structures of covalent network solids and their restricted bond angles are used to describe their properties including rigidity, hardness, brittleness and poor conductivity. Several examples of covalent network solids are covered including diamond, graphite and silicon carbide, which are arranged in a tetrahedral or multi-layered configuration.
74
Molecular Solids - Chemistry
DrOfEng
This chemistry video covers a tutorial on molecular solids. It explains how the intermolecular interactions, including dipoles and london dispersion forces, and the restricted movement of valence electrons are used to describe the properties of molecular solids, including low melting and sublimation points, and poor conductivity. Several examples of molecular solids are covered including dry ice and ice.
75
Metallic Solids - Chemistry
DrOfEng
This chemistry video covers a tutorial on metallic solids. It explains how the lattice structure, which can be represented using a sea of electrons model, and includes delocalised valence electrons, can be used to describe the properties of metallic solids, including malleability, ductility and conductivity of both electricity and heat. An example of an interstitial alloy is provided, being steel, to show how the addition of carbon at the interstitial sites can increase the rigidity while decreasing the ductility of the alloy.
Pure Substances, Percent Composition, Example - AP Chemistry
Enjoyed this video? Join my Locals community for exclusive content at
drofeng.locals.com!
5 months ago
1
This AP Chemistry video covers a worked example on determining the percentage composition of each element in a pure substance.
This video is part of Unit 1 of AP Chemistry on Atomic Structure and Properties.
You can contact me through the comments and community section, or email me at drofeng@gmail.com for questions.
Loading comments...
-
 56:53
56:53
Michael Franzese
1 day agoHuman Trafficking in America is much worse than I thought | Sitdown with Caleb Altmeyer
39.3K38 -
 1:00:57
1:00:57
The StoneZONE with Roger Stone
4 hours agoGrant Cardone: Kamala’s Economic Plans Would Cause a Depression | The StoneZONE with Roger Stone
20.4K7 -

Josh Pate's College Football Show
5 hours agoEp 546: Week 1 Prediction Show | Stalions Netflix Doc | Preseason Rankings Debate | FSU’s Future
13.5K -
 1:05:58
1:05:58
Edge of Wonder
4 hours agoGhostly Orbs Min Min Lights & Marfa Lights: Natural or UFOs?
23.6K3 -
 24:20
24:20
Lauren Chen
3 hours agoBlack People Invented Everything... One Eurasian Woman's Thoughts
27.3K99 -
 38:38
38:38
Chrissy Clark
4 hours agoRacist-In-Chief Robin DiAngelo PLAGIARIZED From Minorities I Underreported Stories
29.2K4 -
 1:08:08
1:08:08
Redacted News
6 hours agoBREAKING! Zuckerberg Admits Biden/Harris Censorship, Tucker Bombshell on JFK Assassination Redacted
110K365 -
 6:35:06
6:35:06
MissesMaam
9 hours agoRed Dead Redemption 2 💚✨
43.5K7 -
 46:28
46:28
Candace Owens
6 hours agoDiddy Accuser Breaks Silence! “He’s A Monster” | Candace Ep 53
54.6K121 -
 16:28
16:28
Megyn Kelly
5 hours agoCorporate Media is Dying While Independent Media is Thriving, w/ Stu Burguiere and Stephen L. Miller
51.6K37