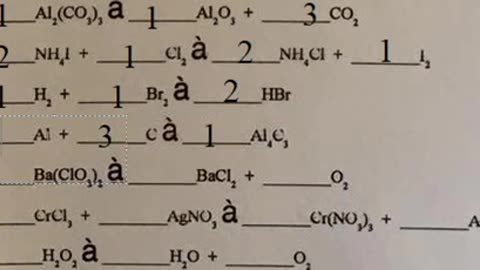Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.

Chemistry Help
saxi753
- 16 / 16
1
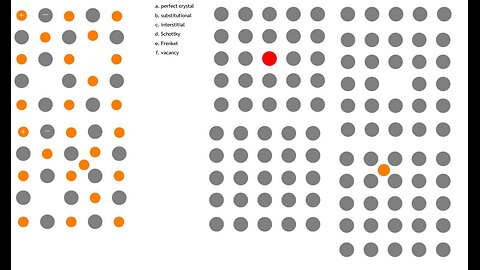
Chemistry: Match the images to the descriptions of point defects: perfect crystal, substitutional
saxi753
Match the images to the descriptions of point defects
perfect crystal
substitutional
interstitial
Schottky
Frenkel
vacancy
2
Chemistry Help: Which of the following directions lie in the (1 1 1) plane?
saxi753
Chemistry Help: Which of the following directions lie in the (1 1 1) plane?
#AtomicArrangments
#ChemistryHelp
3
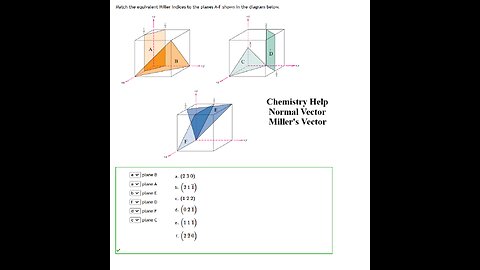
Chemistry Help: Match the equivalent Miller indices to the planes A-F shown in the diagram below.
saxi753
Chemistry Help: Match the equivalent Miller indices to the planes A-F shown in the diagram below.
#MillerPlane
#ChemistryHelp
#OxyzGeometry
#CrossProduct
4
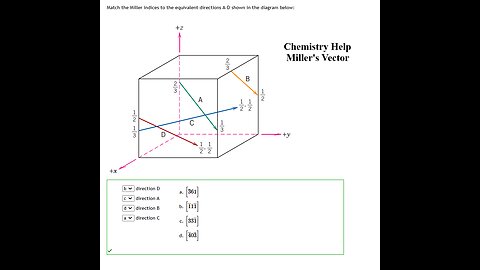
Chemistry Help: Match the Miller indices to the equivalent directions A-D shown in the diagram below
saxi753
Chemistry Help: Match the Miller indices to the equivalent directions A-D shown in the diagram below
#ChemistryHelp
#MillerVector
#Vectors
#OxyzGeometry
5
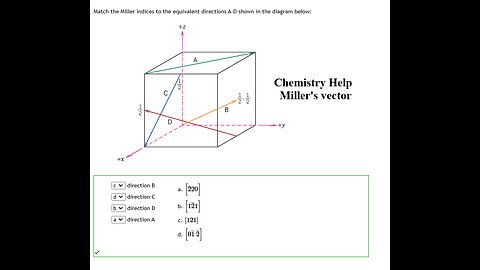
Chemistry Help: Match the Miller indices to the equivalent directions A-D shown in the diagram below
saxi753
#ChemistryHelp
#MillerVector
#Vectors
#OxyzGeometry
6
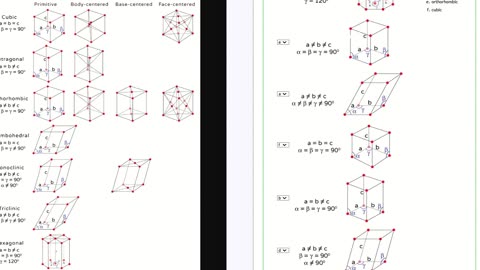
Chemistry Help: Match the unit cell to the name - Full answer with the answer sheet
saxi753
Chemistry Help: Match the unit cell to the name - Full answer with the answer sheet
#ChemistryHelp
#UnitHelp
7
Find the percentage of Oxygen in C2H6O, Find the total mass of Hydrogen in 44 g of C4H8
saxi753
Find the percentage of Oxygen in C_2 H_6 O
Find the total mass of Hydrogen in 44 g of C_4 H_8
Then find the percentage of Hydrogen?
#ChemistryHelp
#Percentage
#Techniques
#Percent
8
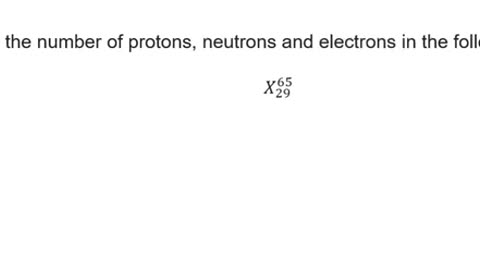
Chemistry Help: Determine the number of protons, neutrons and electrons in the following. X_29_65
saxi753
Here is the technique to illustrate and how to find them
#Proton
#Neutrons
#Electrons
#Chemistry
9
Chemistry Help: Propane C3H8 is bottled as a fuel for barbecues. In 0.500 mol of propane -Molar Mass
saxi753
Here is the technique to do the Molar Mass of organic chemistry
#OrganicChemistry
#MolarMass
10
Chemistry Help: Ratio of moles and grams based on the percentage
saxi753
Question: If a 100g nickel coin is made of 56%Cu, 35%Ag, and 9%Mg,
1. how many MOLES of Cu is in 5g coin?
2. How many GRAMS of Mg is in 5g coin?
#Chemistry
#Technique
#Formula
1
comment
11
Chemistry Help: Proportion and Percent by mass of the elements Glucose,C_6 H_12 O_6
saxi753
Chemistry Help: Proportion and Percent by mass of the elements Glucose,C_6 H_12 O_6
#Proportion
#ChemistryHelp
#Percent
12
Chemistry Help: Valence: How to create chemical compounds in inorganic chemistry
saxi753
Here is the step-by-step about using the valence and how to do chemical compounds
#Chemistry
#Chemical
#Valence
#Techniques
13
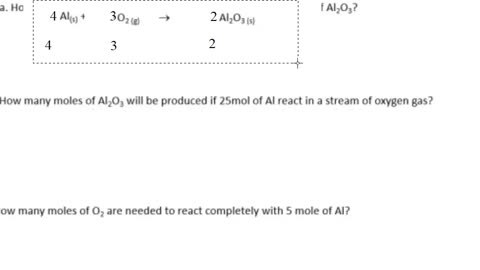
Chemistry Help: Balance the equation, technique to find the moles, the molar mass, the atomic mass
saxi753
Chemistry Help: Balance the equation, technique to find the moles, the molar mass, the atomic mass
#AtomicMass
#Moles
#MolarMass
14
Chemistry Help: Empirical Formula to find the chemical compound by the mass of 100 gram
saxi753
Chemistry Help: Empirical Formula to find the chemical compound by the mass of 100 gram
#EmpiricalFormula
#Empirical
#Chemistry
15
Chemistry Help: Balance all basic chemical reactions (Solutions)
saxi753
Chemistry Help: Balance all basic chemical reactions (Solutions)
#ChemicalReactions
#Techniques
#Solutions
#Balance
Chemistry Help: Suppose a new element with an average atomic mass of 23.187u is
saxi753
Suppose a new element with an average atomic mass of 23.187u is discovered on Mars. This element is composed of two isotopes. The most abundant isotope has a natural abundance of 77.740% and an isotopic mass of 23.088 u. Calculate the isotopic mass of the least abundant isotope
Chemistry Help: Suppose a new element with an average atomic mass of 23.187u is
10 months ago
24
Suppose a new element with an average atomic mass of 23.187u is discovered on Mars. This element is composed of two isotopes. The most abundant isotope has a natural abundance of 77.740% and an isotopic mass of 23.088 u. Calculate the isotopic mass of the least abundant isotope
Loading comments...
-
 2:02:18
2:02:18
Side Scrollers Podcast
1 day agoVShojo COLLAPSES, Unhinged CELEBRATE Hulk Hogan’s Death, Chuck E Cheese ARRESTED | Side Scrollers
46.2K7 -
 LIVE
LIVE
BatDude Gaming
2 hours ago🦇 RUMBLE GAMING 🦇 ⚡⚡ TALES from the BORDERLANDS (Continued) ⚡⚡ STORY BASED GAME 🦇
24 watching -
 LIVE
LIVE
Rotella Games
3 hours agoSaturday Morning Family Friendly Fortnite
100 watching -
 2:17:51
2:17:51
HartZA92
3 hours agoGenesis Alpha One: Build, Blast & Survive the Final Frontier!
16.9K1 -
![[HD] Gamer Stream](https://1a-1791.com/video/fww1/53/s8/1/c/G/G/5/cGG5y.Gkob-small-HD-Gamer-Stream.jpg) LIVE
LIVE
darealchurchiee
4 hours ago[HD] Gamer Stream
75 watching -
 3:53:47
3:53:47
Anvilight
6 hours agoWorld of Warcraft | Trump Arrives in Scotland to Meet with Anvilight for Azeroth Peace Negotiations
13.2K1 -
 25:49
25:49
GritsGG
15 hours agoRank 1 Player Crushes Solo Lobby!
36.9K3 -
 3:33:15
3:33:15
Wahzdee
3 hours ago🔥 Trying to Find The One Extraction Game – Be Honest, Are These Fun to Watch?
12.6K -
 35:14
35:14
The Pascal Show
16 hours ago $8.52 earnedHE'S GONNA SUE COLDPLAY?! Astronomer HR Resigns & Ex-CEO Set To Sue Coldplay Over Kiss Cam Drama
40.3K17 -
 LIVE
LIVE
Lofi Girl
2 years agoSynthwave Radio 🌌 - beats to chill/game to
273 watching