How To Balance Redox Equations In Basic Solution
7 months ago
61
Science
Education
how to balance redox reactions in basic solution
redox reactions in basic solution
how to balance redox reactions
redox reactions basic solution
redox reactions balancing
redox reactions
basic solution
redox
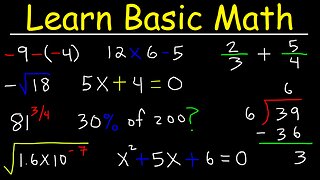
This chemistry video tutorial shows you how to balance redox reactions in basic solution. The first step is to separate the net reaction into two separate half reactions - Oxidation and Reduction. Balance the atoms first under acidic conditions using H+ and H2O and then balance the charges by adding electrons to the side of the chemical equation with the highest oxidation state. Once the electrons in both half-reactions are equal, the two reactions may be combined together to form the net reaction. Add OH- ions to both sides of the equation to neutralize the acid - this is how you can balance the redox reaction under basic conditions.
Loading 1 comment...
-
 1:12:08
1:12:08
TheOrganicChemistryTutor
7 months agoMath Videos: How To Learn Basic Arithmetic Fast - Online Tutorial Lessons
761 -
 4:45
4:45
Gamazda
19 hours agoDragonForce - Through the Fire and Flames (Piano)
3.51K19 -
 24:56
24:56
MYLUNCHBREAK CHANNEL PAGE
10 hours agoFinding Old World Pyramids?
6.19K35 -
 16:20
16:20
Guns & Gadgets 2nd Amendment News
10 hours agoClarence Thomas DESTROYS Opinion Of Today's Anti-2A Ruling!.mp4
4.07K9 -
 26:42
26:42
Degenerate Plays
9 hours agoHeavenly Bodies And Beautiful Assets - Stellar Blade : Part 1
4K1 -
 7:03
7:03
Chris Jericho
1 day agoTalk Is Jericho Highlight: Alien Encounters With Mitch Horowitz
3.48K1 -
 3:01:45
3:01:45
SNEAKO
5 hours agoSUMMER IS HERE… SPECIAL GUEST
57K34 -
 1:07:00
1:07:00
Russell Brand
1 day ago“Something BIG is about to happen” - EXCLUSIVE Alex Jones Interview on INFOWARS shut down - 391
426K2.36K -
 2:06:37
2:06:37
Steven Crowder
2 days agoREBUTTAL: Jon Stewart is WRONG about Gun Violence
517K899 -
 LIVE
LIVE
Right Side Broadcasting Network
7 days agoLIVE: President Trump Holds a MAGA Rally in Philadelphia - 6/22/24
5,309 watching