The atomic arrangement of each crystalline material can be divided into six types
1 year ago
4.11K
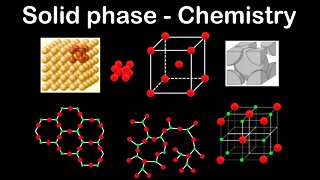
Each crystalline material’s atomic arrangement falls into one of six different families: cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal. Given the appropriate conditions, crystals will grow into geometric shapes that reflect the arrangement of their atoms. Take galena, which has a cubic structure composed of lead and sulfur atoms. The relatively large lead atoms are arranged in a three-dimensional grid 90 degrees from one another, while the relatively small sulfur atoms fit neatly between them.
Loading comments...
-
 7:40
7:40
Center for Metaphysical Ecology
1 year agoThe Crystalline Universe
28 -
 4:08
4:08
OmegaOrdained
3 months agoThe Crystalline Entity
22 -
 4:07
4:07
Chemistry - DrOfEng
1 year agoSolid phase, crystalline, amorphous - Chemistry
9 -
 0:53
0:53
NataliaNatalia0
1 year agoWater exposed to 5G - Crystallography -
46 -
 6:31
6:31
Center for Metaphysical Ecology
1 year agoWorking with Crystalline energies
9 -
 0:27
0:27
JodyAlbertMaas
2 years agoOrganizing my crystals
20 -
 0:40
0:40
LaOtaOfTheSun
6 months agoWe Are Crystalline!
7 -
 54:23
54:23
spbwsu
3 years agoCrystallography and Diffraction
10 -
 16:32
16:32
ME 3450: Properties of Materials
3 years agoCeramics - Crystal Structure (Part 2 of 2)
23 -
 10:37
10:37
ME 3450: Properties of Materials
3 years agoCeramics - Crystal Structure (Part 1 of 2)
43