Balancing Chemical Equations Video: Are Reactants and Products Mass Balanced? (Lecture 5)
Balancing chemical equations (balancing equations) is a very important skill that all chemistry students must master. Before working with any chemical equation it must be mass balanced in order to obey law of conservation matter (law of conservation of mass). The law of conservation of mass states that in an chemical reaction matter cannot be created nor destroyed. Therefore, the matter (mass) on the reactant side must equal the matter (mass) on the product side of the equation. This translates into the atoms on the left of the arrow must equal the atoms on the right of the arrow. For example, if on the left of the arrow there are 3 oxygen atoms then there has to be three oxygen on the right of the arrow.
A common question on chemistry exams is:
balance the following chemical equation followed by an unbalanced chemical equation.
This video will discuss
law of conservation of mass
chemical equations
balancing chemical equations (balancing equations, mass balancing)
law of conservation of matter(conservation of mass, conservation of matter)
balancing chemical equations practice
mass balance equation
chemical equation examples
balancing equations practice
balancing equations examples
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below! https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
#nursing
#prenursing
#prenurse
#prenursingchem
#prenursingchemistry
#balancingequations
#lawofconservationmatter
#massbalance
-
 0:58
0:58
Chemistry - DrOfEng
1 year ago $0.04 earnedBalancing chemical equations, example - Chemistry
36 -
 4:08
4:08
Chemistry - DrOfEng
1 year agoBalancing chemical equations, example - Chemistry
2 -
 1:51
1:51
Chemistry - DrOfEng
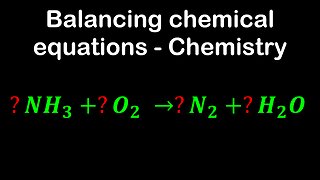
1 year agoBalancing chemical equations - Chemistry
9 -
 11:19
11:19
CuriousPeasant
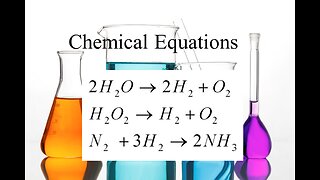
6 months agoChem003a_chemical_equations
14 -
 54:35
54:35
DTM High School Chemistry
1 year agoUnit 6 Recording 2 Prediction of Products and Balancing Chemical Equations
3 -
 3:39
3:39
Chemistry - DrOfEng
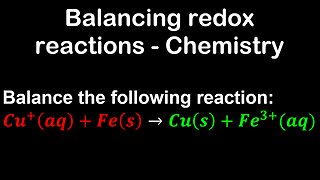
1 year ago $0.04 earnedBalancing redox reactions, example - Chemistry
28 -
 8:07
8:07
Chemistry - DrOfEng
1 year agoBalancing redox reactions, basic solution, example - Chemistry
9 -
 15:44
15:44
TheOrganicChemistryTutor
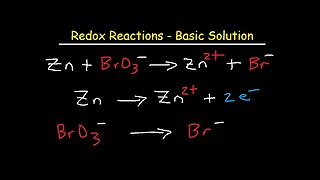
7 months agoHow To Balance Redox Equations In Basic Solution
611 -
 7:06
7:06
Chemistry - DrOfEng
1 year ago $0.01 earnedBalancing redox reactions, acidic solution, example - Chemistry
4 -
 0:57
0:57
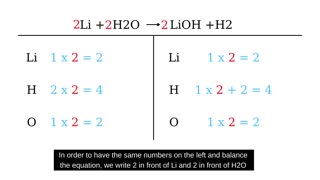
ChemEasy
3 years ago $0.19 earnedHow to balance Li+H20=LiOH+H2
455