Premium Only Content
Elements
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#elements #AtomicNumber #Chemistry
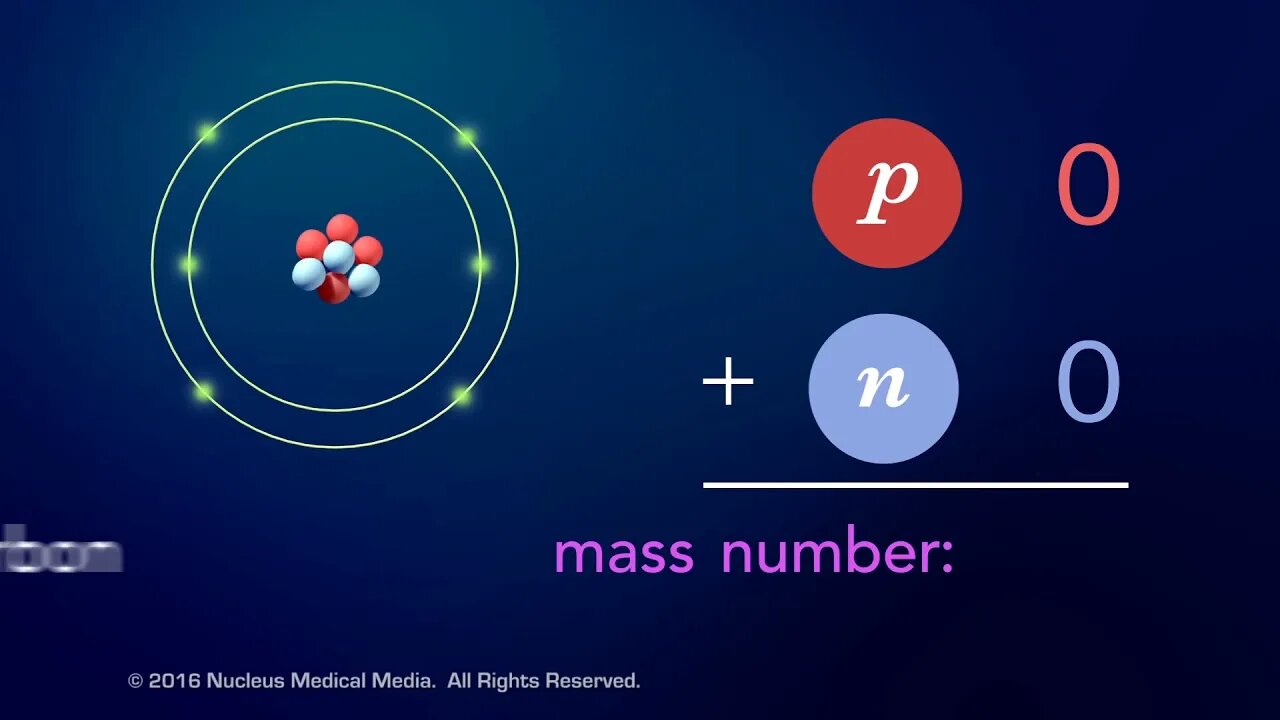
SCIENCE ANIMATION TRANSCRIPT: What is an element? Elements are pure substances that are made up of only one type of atom such as hydrogen, carbon, or mercury. So, what makes one element different from another? Well, it's the number of protons in a single atom of an element. This is called the element's atomic number. For example, hydrogen has one proton in its nucleus, so its atomic number is one. Carbon has six protons, so its atomic number is six. And mercury's atomic number is 80 because it has 80 protons in its nucleus. Since the nucleus contains almost the entire mass of an atom, the number of particles it contains has a big effect on the atom's mass. Each positively charged proton has a mass unit of one, and each neutral neutron also has a mass unit of one. The total number of protons and neutrons in the nucleus of an atom is called the mass number. In this example, the mass number of a hydrogen atom is one. Hydrogen is the only element that usually doesn't have any neutrons. The mass number of this carbon atom with six protons and six neutrons is 12. And the mass number of this mercury atom with 80 protons and 121 neutrons is 201. Needless to say, mercury is a match heavier element than hydrogen or carbon. Even though every atom of the same element always has the same number of protons, sometimes an element has atoms with different numbers of neutrons. Ordinary hydrogen has no neutrons, but there's a version of hydrogen with one neutron and another version with two neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. The three isotopes you see here are all still hydrogen because they all have only one proton. Since neutrons have about the same mass as protons, isotopes of the same element have different mass numbers. In fact, an element's isotopes are often identified by their mass numbers. To sum up, an element is a pure substance made of atoms that always have the same number of protons. This means atoms with different numbers of protons are different elements. The number of protons in one atom of an element is called the atomic number. The number of protons plus neutrons in one atom is called the mass number. Isotopes are atoms of the same element with different numbers of neutrons. [music]
NSV16021
-
 12:40
12:40
Tundra Tactical
13 hours ago $2.31 earnedGEN Z Brit 3D Prints a WORKING Gun Pt.2!
14.9K9 -
 10:45
10:45
The Rich Dad Channel
19 hours agoWhy Working Hard Will Keep You Poor (Unless You Do This)
11.4K2 -
 16:32
16:32
Melonie Mac
16 hours agoPhoebe Waller Bridge fails to deliver Tomb Raider script
16.8K4 -
 17:19
17:19
ARFCOM Reviews
18 hours ago $0.55 earnedComplete SI Pistol Build | Strike Arms Compact Pistol
12.3K1 -
 1:08:43
1:08:43
MTNTOUGH Fitness Lab
14 hours agoInside the WILDEST Career Switch: How a Ballroom Dancer Conquered the Hunting World
18.1K4 -
 26:57
26:57
Uncommon Sense In Current Times
16 hours ago $0.53 earnedDefending Your Christianity Without Overcomplicating It (Part 1) | Greg Koukl
14.8K3 -
 52:58
52:58
State of the Second Podcast
13 hours agoThe Unfiltered HONEST Truth About the Firearms Community (ft. Whiskey & Windage)
17.2K4 -
 2:22:54
2:22:54
Badlands Media
15 hours agoBad Friends Ep. 1: In the Beginning Was the Word... and a Lot of Chaos
87.1K73 -
 2:57:33
2:57:33
TimcastIRL
12 hours agoFBI CAUGHT Rigging 2020 Election, Leaked Chat Logs PROVE COVER UP w/Michael Malice | Timcast IRL
276K225 -
 2:30:03
2:30:03
The Quartering
12 hours agoWisconsin Supreme Court Race RESULTS Brad Schimel Vs Susan Crawford!
124K59