Premium Only Content
Overview of Chemical Bonds
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#ChemicalBonds #IonicBonds #CovalentBonds
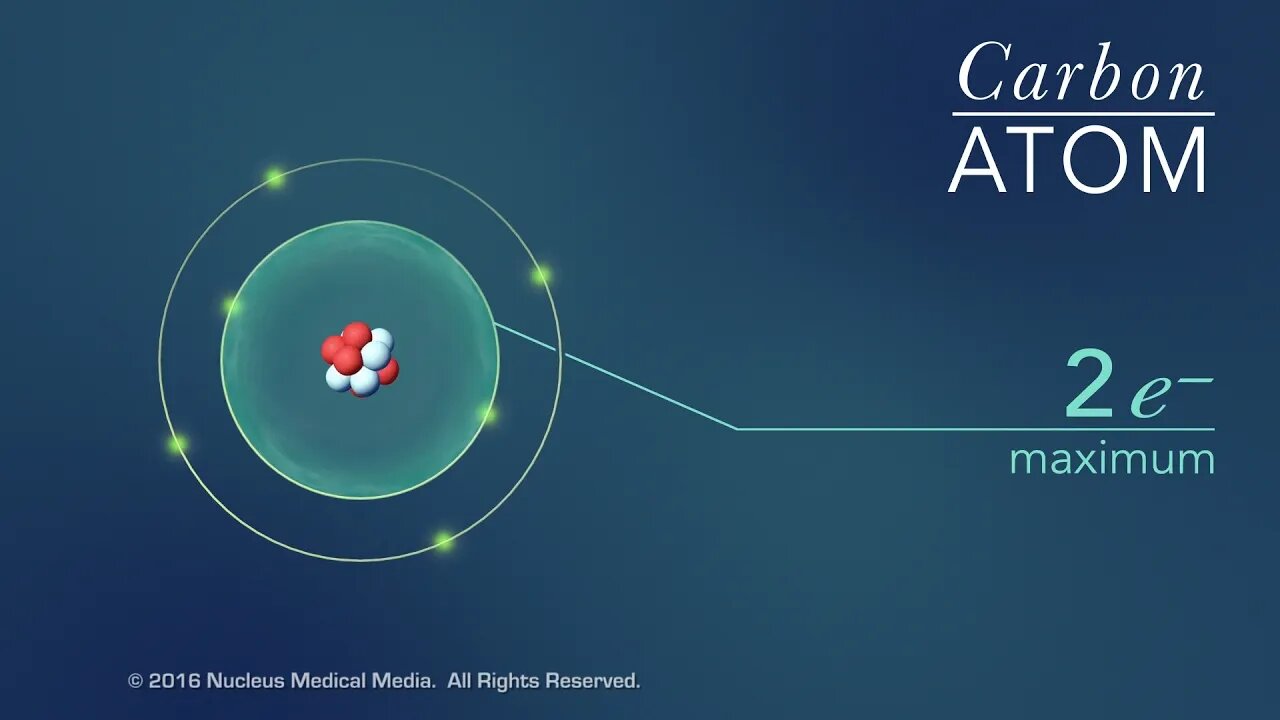
SCIENCE ANIMATION TRANSCRIPT: This video is an overview of chemical bonds. How do the atoms of elements form chemical bonds? Recall that electrons and an atom surround the nucleus. They feel energy levels or shells in specific numbers. Electrons in an atom's inner shells are commonly referred to as core electrons. Core electrons don't participate in chemical bonds. In contrast, electrons in the outermost shell of an atom are called valence electrons. Valence electrons do participate in forming chemical bonds. For example, a carbon atom with six protons and six neutrons in the nucleus has six electrons. Notice that carbon has both core and valence electrons. The innermost shell of any atom can hold a maximum of two electrons and the next shell can hold up to eight electrons. So, two of the electrons in carbon fill the first shell, and the remaining four electrons are in the next shell. The two electrons in the first shell are carbon's core electrons. The four electrons in the outer shell are carbon's valence electrons. Atoms with fewer valence electrons than its outer shell can hold aren't as stable as atoms with full outer shells. However, these atoms can become more stable if their outer shell is filled. This can happen either by loosing electrons to another atom or attracting electrons from another atom. This interaction of valence electrons between atoms results in the formation of chemical bonds. Elements that have completely filled outer shells such as helium are mostly non-reactive which means, they don't usually form chemical bonds. Why is that? Well, it's because their outer shells can't accept anymore electrons and losing all of their valence electrons requires too much energy. There are two main types of chemical bonds, they are ionic bonds, when electrons are transferred from one atom to another, and covalent bonds, when atoms share electrons. We'll discuss this in more detail separately. [music]
NSV16020
-
 1:59:06
1:59:06
The Charlie Kirk Show
2 hours agoBlackpilled Zoomers + Charlie's Sabbath + Minnesota Meltdown | Bowyer, Turek, Glahn | 12.3.2025
47.7K14 -
 12:44
12:44
Dr. Nick Zyrowski
2 days agoThe REAL Benefits of One Meal A Day (OMAD) Intermittent Fasting
8.57K1 -
 2:30:50
2:30:50
Tucker Carlson
1 hour agoWhy Are You Gay? Milo Yiannopoulos Explains.
21.3K93 -
 LIVE
LIVE
Barry Cunningham
16 hours agoLIVE BREAKING NEWS: President Trump Addresses The Nation | Marco Rubio | Kash Patel | News!
1,989 watching -
 59:34
59:34
Timcast
2 hours agoNarco Boat Double Strike Triggers MASSIVE Political Firestorm, Admiral To Be QUESTIONED By Congress
132K72 -
 2:03:59
2:03:59
Side Scrollers Podcast
4 hours agoKaceytron Publicly Humiliated by H3H3 + Sabrina Carpenter/White House FEUD + More | Side Scrollers
30.8K4 -
 5:58
5:58
Buddy Brown
5 hours ago $1.63 earnedAngel CAUGHT ON CAMERA Saves Street Preacher from ATTACK! | Buddy Brown
15.9K13 -
 LIVE
LIVE
SternAmerican
1 day agoELECTION INTEGRITY CALL – WED, DEC 3 AT 2 PM EST | FEATURING TEXAS
125 watching -
 2:01:57
2:01:57
Steven Crowder
5 hours agoVintage MAGA: Trump's Epic Somalia Rant Isn't Racist - It's Irrefutable
365K264 -
 12:23
12:23
The Illusion of Consensus
4 hours ago $0.30 earnedThe Moment Dave Smith was Accused of Being A Holocaust Denier by Alex Berenson
13.9K2