Premium Only Content
Overview of Organic Compounds
For Employees of hospitals, schools, universities and libraries: download up to 8 FREE medical animations from Nucleus by signing up for a free trial at: http://nmal.nucleusmedicalmedia.com/biology_youtube
#OrganicCompounds #OrganicMonemers #polymers
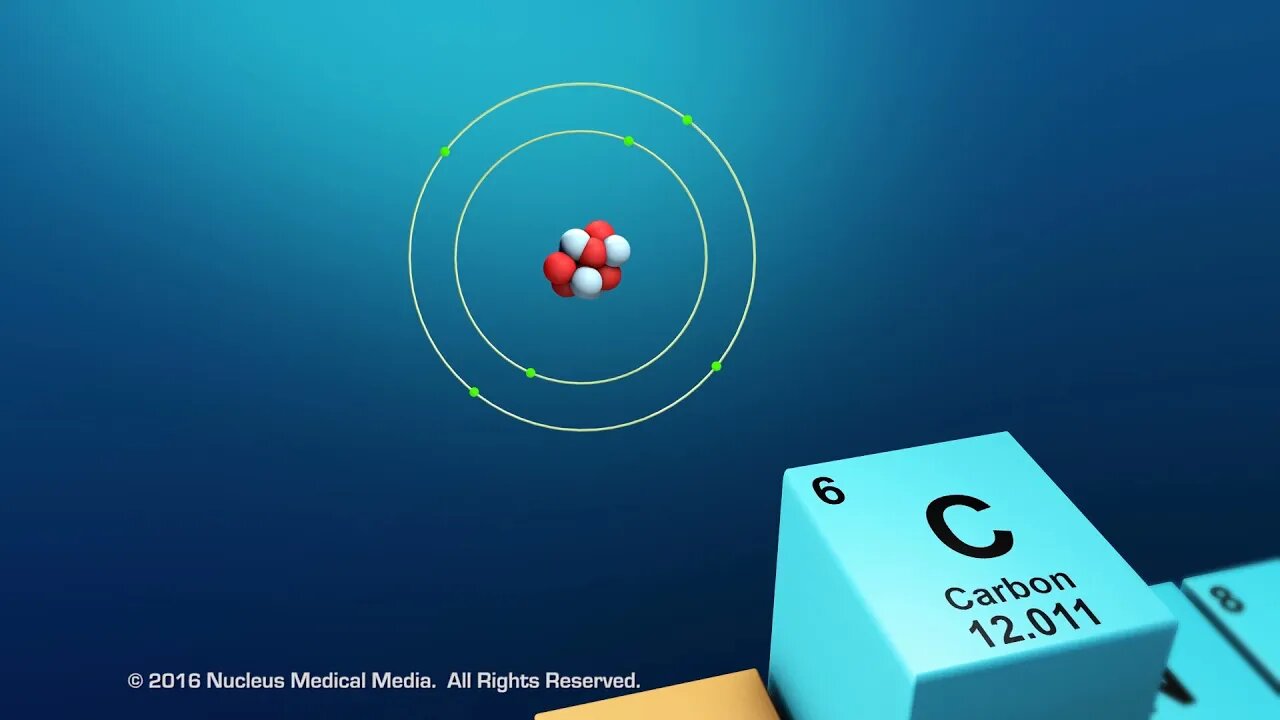
SCIENCE ANIMATION TRANSCRIPT: Today, we are going to be talking about organic compounds. Organic compounds are studied in biology because they are found in all living things. All organic compounds have the element carbon in them. In other words, all living organisms contain carbon. What's so special about the element, carbon? For one thing, no other element matches carbon's unique versatility to bond with other elements. Let's look at why this is true. For starters, carbon has an atomic number of six. That means that every atom of carbon has six protons in the nucleus. As an electrically neutral atom, carbon also has six electrons. Two core electrons are in the first energy level, which means it has four remaining valence electrons in the second energy level. Remember, valence electrons are those electrons available for bonding with other atoms. Accompanying these four electrons are four bonding sites, or four places that carbon can form bonds with other carbon atoms, or with atoms of other elements. Carbon's four valence electrons and four bonding sites allow it to form strong covalent bonds with many other elements, including hydrogen, oxygen, nitrogen, and phosphorus. Another feature of carbon atoms is they often form covalent bonds with other carbon atoms, to a nearly unlimited degree. This means that two carbon atoms can bond to one another, 50 carbon atoms can bond to one another, or even hundreds of carbon atoms can bond to one another. The ability of carbon atoms to bond to one another gives it the unique ability to shorten or lengthen a chain of carbon atoms to meet the very demands of the chemistry of life. So what kinds of molecules can carbon form? Well, small organic molecules called monomers are chemically bonded atoms that always include carbon. In addition to carbon, organic monomers usually contain hydrogen and oxygen, possibly along with nitrogen or phosphorus. Organic monomers often chemically bond to each other, joining together like beads on a string. This string of attached monomers will often continue to chemically bond with additional monomers, creating a much larger molecule, called a polymer. This process is called polymerization. Polymers may be made of different monomers or repeating units of the same monomer. Many organic polymers in the cells of living organisms are such large molecules that they're often referred to as macromolecules. DNA is an example of a macromolecule. Macro molecules can contain hundreds or even thousands of atoms. The four types of organic macromolecules are carbohydrates, lipids, proteins, and nucleic acids. Although they are all very large molecules, each type of organic macromolecule is distinct and different from the others. We'll discuss these four types of macromolecules in more detail separately. To sum up, organic compounds are found in all living things. All organic compounds contain the element carbon. Carbon atoms have a unique ability to bond to other carbon atoms, as well as other elements, such as hydrogen, oxygen, nitrogen, and phosphorus. Organic monomers are chemically bonded atoms that always include carbon. Polymerization is the process of creating long molecules, called polymers, from multiple bonded monomers. And macromolecules are very large organic molecules. [music]
NSV16027
-
 LIVE
LIVE
Mally_Mouse
1 day ago🔥🍺Spicy HYDRATE Saturday!🍺🔥-- Let's Play: Grounded
69 watching -
 24:09
24:09
MYLUNCHBREAK CHANNEL PAGE
1 day agoDams Destroyed The Ozarks
60.6K29 -
 1:32:54
1:32:54
Jeff Ahern
5 hours ago $24.39 earnedThe Saturday Show with Jeff Ahern
83.3K9 -
 LIVE
LIVE
TheManaLord Plays
7 hours agoMANA SUMMIT - DAY 1 ($10,200+) | BANNED PLAYER SMASH MELEE INVITATIONAL
208 watching -
 LIVE
LIVE
Major League Fishing
2 days agoLIVE Tackle Warehouse Invitationals Championship, Day 2
117 watching -

GamerGril
4 hours agoScream Queens 💕 Goth & Gore 💕 Unpossess
17.1K2 -
 LIVE
LIVE
CassaiyanGaming
8 hours agoMYSTIVITHON - 12 HOUR CHARITY STREAM 🌊
68 watching -
 2:14:16
2:14:16
Lara Logan
20 hours agoSTANDING AGAINST THE GLOBAL ELITE with Trump Ally President Milorad Dodik of Republika Srpska | Ep34
38.8K25 -

FoeDubb
6 hours ago🏰KINGDOM MENU: 👑CHILL CONVO 🎮DELTA FORCE PEW PEWS WITH THE HOMIES ARROWTHORN & JFGSTEVEN
12.6K1 -
 1:56:00
1:56:00
PixiEars_
4 hours agoBDAY STREAM
9.85K2