Premium Only Content
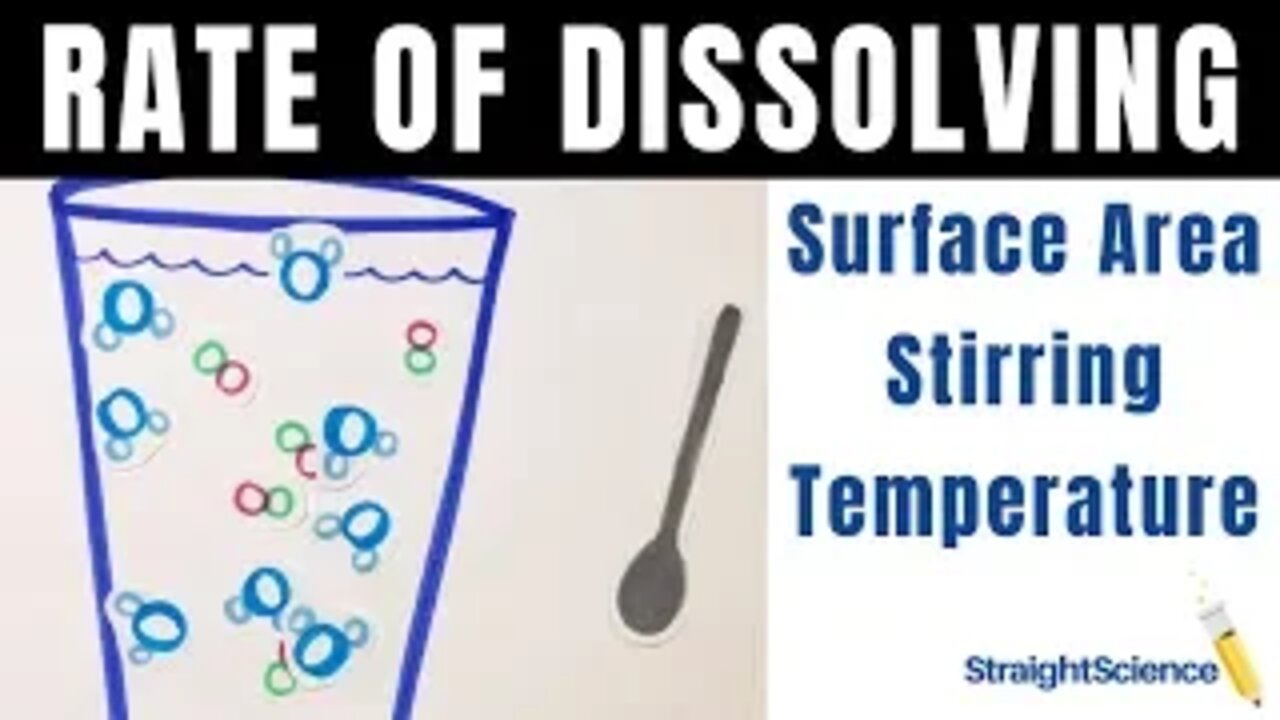
Rate of Dissolving - Increase the Rate - Surface Area - Stir - Temperature
Okay! In this video we look at how to increase the RATE a solute dissolves into a solvent. Please make sure you understand that the only thing we are affecting is how fast the solute dissolves, NOT how much solute dissolves. If you want to see how much solute will dissolve, check out the solubility curve.
If you want to understand how to get a solute to dissolve faster, watch on. There are three ways increase the rate of dissolving:
1. Increase the Surface Area -
individual grains of a solute will dissolve faster than a giant cube of the solute because the water molecules are actually able to surround the solute particles. The more surface area a solute has (the smaller the particles) the faster it will dissolve.
2. Stir the Solution -
If you stir the solution, you are causing the solute and solvent particles to move faster and collide with each other more often. If a solvent and solute collide more often and at a higher speed, the likelihood that the solute will dissolve in that solvent is increased.
3. Increase the Temperature -
Temperature is just a measurement of the kinetic energy of a substance (how fast the particles are moving). If you increase the temperature of a substance, the kinetic energy is increased, meaning that those particles are moving faster. If you increase the temperature of a solution, the solvent and solute particles are moving much faster. This means they are colliding into each other more often and faster, making the solute dissolve faster into that solvent.
Good luck!
-
 7:54:17
7:54:17
Putther
10 hours ago $5.06 earned🔴LAZY SUNDAY STREAM!! (GTA + MORE)
48.3K9 -
 10:38
10:38
Colion Noir
3 hours agoHe Installed a Forced Reset Trigger at a Gun Range… and Got Arrested | What You Need to Know
42.9K15 -
 1:29:26
1:29:26
HELMETFIRE
3 hours ago🟢GAMING WITH FIRE EP13🟢
8.55K3 -
 50:40
50:40
Sarah Westall
5 hours agoAI, Social Media & Brain Atrophy: Destroying Human Capacity to Think w/ Rob Smith
16.5K6 -
 LIVE
LIVE
THOUGHTCAST With Jeff D.
2 hours ago $0.01 earnedSunday Night FORTNITE with THOUGHTCAST Jeff D & crew.
314 watching -
 55:10
55:10
The Mel K Show
10 hours agoMel K & Mike L | The Tylenol Piece: It's a Marathon, Not a Sprint | 10-19-25
80.5K11 -
 3:17:37
3:17:37
MrMoBetta13
3 hours agoCFB26 CUT Ranked + NEW NIGHTMARE CARDS and talking college football!!
8.36K2 -
 LIVE
LIVE
Spartan
6 hours agoOMiT Spartan | God of War Ragnarok and then Halo
66 watching -
 3:19:39
3:19:39
FusedAegisTV
1 day agoλ Black Mesa λ (Half Life 1 Remake) █ Western Retread
14.2K1 -
 40:22
40:22
MattMorseTV
4 hours ago $0.27 earned🔴They just tried it... AGAIN.🔴
22.1K56