Premium Only Content
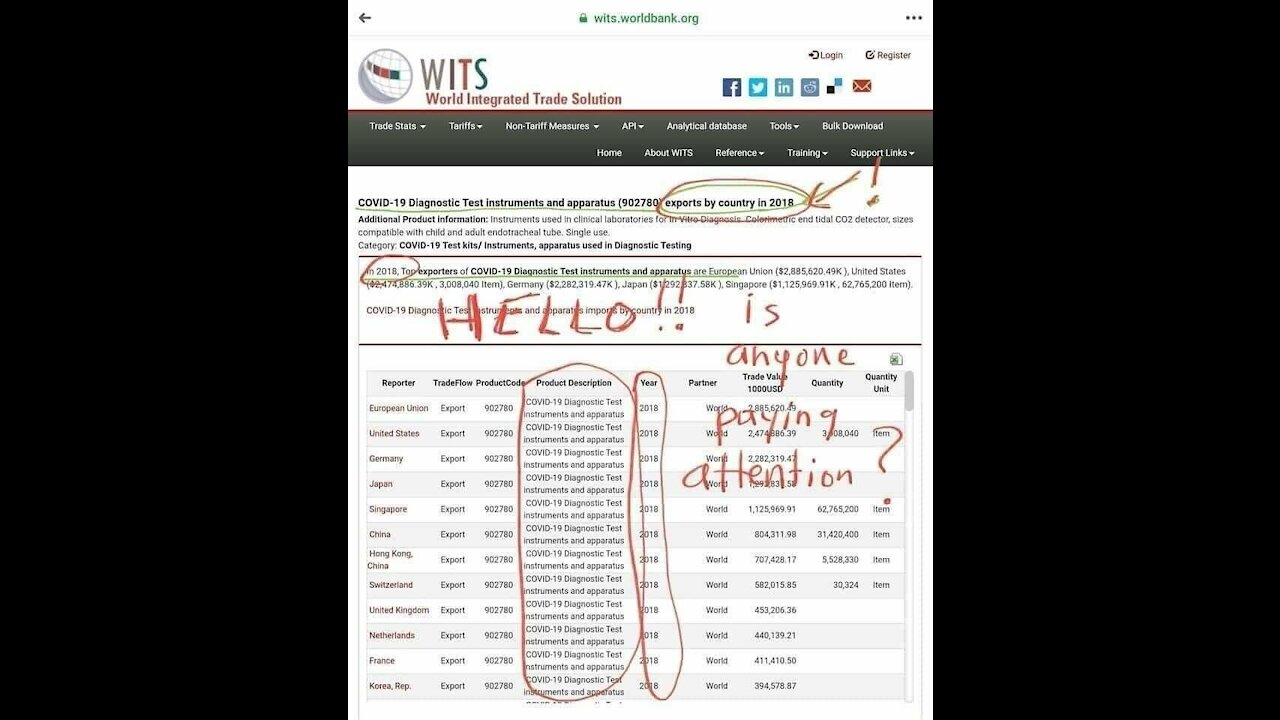
OUR GOVERNMENT Purchased C-19 Testkits WHEN????
Today we look at the governments of the world. Is it a conspiracy when you have the paperwork...receipts...verifying what you claim???
We aslo look at a ibe some mectin alternatives...yes Rumble does have censor-algorithms. Why else would child trafficking productions aimed at creepy uncle joe be removed without notice...Still waiting for an answer Mr. Bongino...
https://www.newsweek.com/exclusive-secret-commandos-shoot-kill-authority-were-capitol-1661330
https://pubmed.ncbi.nlm.nih.gov/34407441/
Fair Use Notice:
Use the information found in the videos as a starting point for conducting your own research and conduct your own due diligence before making any significant investing decisions.
Under the following terms", Attribution-NonCommercial-ShareAlike 4.0 International.
https://creativecommons.org/licenses/by-nc-sa/4.0/
https://creativecommons.org/licenses/by-nc-sa/4.0/legalcode
As with all Independent Journalist folks we can use a helping hand. If you can make a contribution to assist in keeping the lights on please go to my website and use the donate feature in the right sidebar. Thank you
https://paypal.me/aaronaveiro1?locale.x=en_US
Please visit our website and let us know what you think. You may also use the paypal features for donations or other inquiries.
https://aladaymobilemedia.com
Abstract
Background: Effective treatment for Coronavirus Disease-2019 (COVID-19) is under intensive research. Nigella sativa oil (NSO) is a herbal medicine with antiviral and immunomodulatory activities, and has been recommended for the treatment of COVID-19. This study aimed to evaluate the efficacy of NSO treatment in patients with COVID-19.
Methods: All adult patients with mild COVID-19 symptoms presented to King Abdulaziz University Hospital, Jeddah, Saudi Arabia, were recruited for an open label randomized clinical trial (RCT). They were randomly divided into control or treatment groups, with the latter receiving 500 mg NSO (MARNYS® Cuminmar) twice daily for 10 days. Symptoms were daily monitored via telecommunication. The primary outcome focused on the percentage of patients who recovered (symptom-free for 3 days) within 14-days. The trial was registered at clinicaltrials.gov (NCT04401202).
Results: A total of 173 patients were enrolled for RCT. The average age was 36(±11) years, and 53 % of patients were males. The control and NSO groups included 87 and 86 patients respectively. The percentage of recovered patients in NSO group (54[62 %]) was significantly higher than that in the control group (31[36 %]; p = 0.001). The mean duration to recovery was also shorter for patients receiving NSO (10.7 ± 3.2 days) compared with the control group (12.3 ± 2.8 days); p = 0.001.
Conclusions: NSO supplementation was associated with faster recovery of symptoms than usual care alone for patients with mild COVID-19 infection. These potential therapeutic benefits require further exploration with placebo-controlled, double-blinded studies.
Keywords: COVID-19; Herbal medicine; Nigella sativa; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
-
 55:02
55:02
Bek Lover Podcast
16 hours agoAl Qaeda Take Over of Syria Backed by US & Israel? More Strange News...
6.34K2 -
 4:04:32
4:04:32
Alex Zedra
7 hours agoLIVE! New Scary Game w/ Heather
113K3 -
 49:19
49:19
barstoolsports
12 hours agoThe Game is Officially On | Surviving Barstool S4 Ep. 5
126K2 -
 4:33:30
4:33:30
BSparksGaming
8 hours agoYou're Next FAVORITE Rumble Streamer! Hump Day BO6 Grind! #RumbleTakeover
38.7K2 -
 3:22:22
3:22:22
Pepkilla
9 hours agoCan we get to Silver II on ranked toniiiight ~
30.6K1 -
 5:00:49
5:00:49
Drew Hernandez
8 hours agoPROJECT BLUE BEAM OR IRANIAN DRONES?
49.6K30 -
 1:42:58
1:42:58
Kim Iversen
11 hours agoEvacuating My Christian Family from Al-Qaeda-Controlled Syria: Kevork Almassian | Trump To End Birthright Citizenship? Jamarl Thomas
142K61 -
 33:31
33:31
Stephen Gardner
8 hours ago🔴JUST IN: DA Alvin Braggs THREATENS Trump | Canada Justin Trudeau OFFENDS Americans!
77.6K199 -
 2:25:09
2:25:09
Barry Cunningham
9 hours agoThe Evening News: Chris Wray Resignation Has Media In FREAKOUY Mode!
83.9K29 -
 1:49:35
1:49:35
I_Came_With_Fire_Podcast
13 hours agoNEW JERSEY UAP/DRONES—What are they!?
34.4K10