Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
Vaccines and Related Biological Products Advisory Committee – 9/17/2021 (Mirror)
3 years ago
83
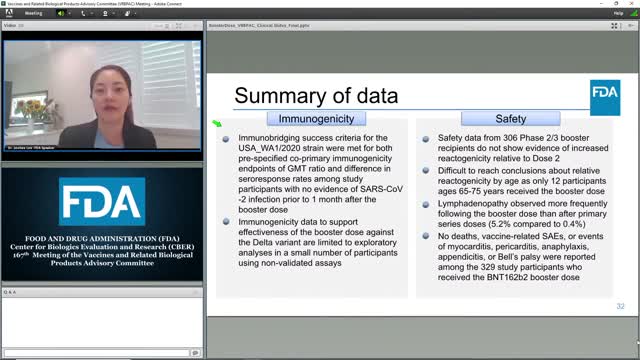
Join us for a Vaccines and Related Biological Products Advisory Committee meeting to discuss Pfizer-BioNTech’s supplemental Biologics License Application for administration of a third dose, or “booster” dose, of the COVID-19 vaccine, Comirnaty, in individuals 16 years of age and older.
Loading comments...
-
 LIVE
LIVE
LFA TV
12 hours agoLFA TV ALL DAY STREAM - MONDAY 8/25/25
5,240 watching -
 LIVE
LIVE
Welcome to the Rebellion Podcast
18 hours agoMonday Funday - WTTR Podcast Live 8/25
536 watching -
 LIVE
LIVE
Game On!
14 hours agoTom Brady And The Las Vegas Raiders ARE BACK! 2025 NFL Preview!
7,179 watching -
 LIVE
LIVE
The Bubba Army
2 days agoShould RaJa Jackson Be Arrested? - Bubba the Love Sponge® Show | 8/25/25
3,173 watching -
 LIVE
LIVE
FyrBorne
13 hours ago🔴Warzone M&K Sniping: Builds So Strong They Think I'm Hacking
511 watching -
 LIVE
LIVE
BEK TV
2 days agoTrent Loos in the Morning - 8/25/2025
1,681 watching -
 4:23
4:23
Blackstone Griddles
16 hours agoEasy Salmon Dinner on the Blackstone Griddle
27.3K1 -
 8:10
8:10
WhaddoYouMeme
1 day ago $0.06 earnedChristians, Before You See “Testament”, Watch this!
7.36K4 -
 8:42
8:42
Freedom Frontline
14 hours agoDurbin’s Trump Smear Video Just HUMILIATED Him in the Senate
9.21K4 -
 10:56
10:56
ariellescarcella
12 hours agoThe Shocking Divide Among College Voters Sparks Worry For America
7.57K6