Premium Only Content
How vaccines work against COVID-19: Science, Simplified
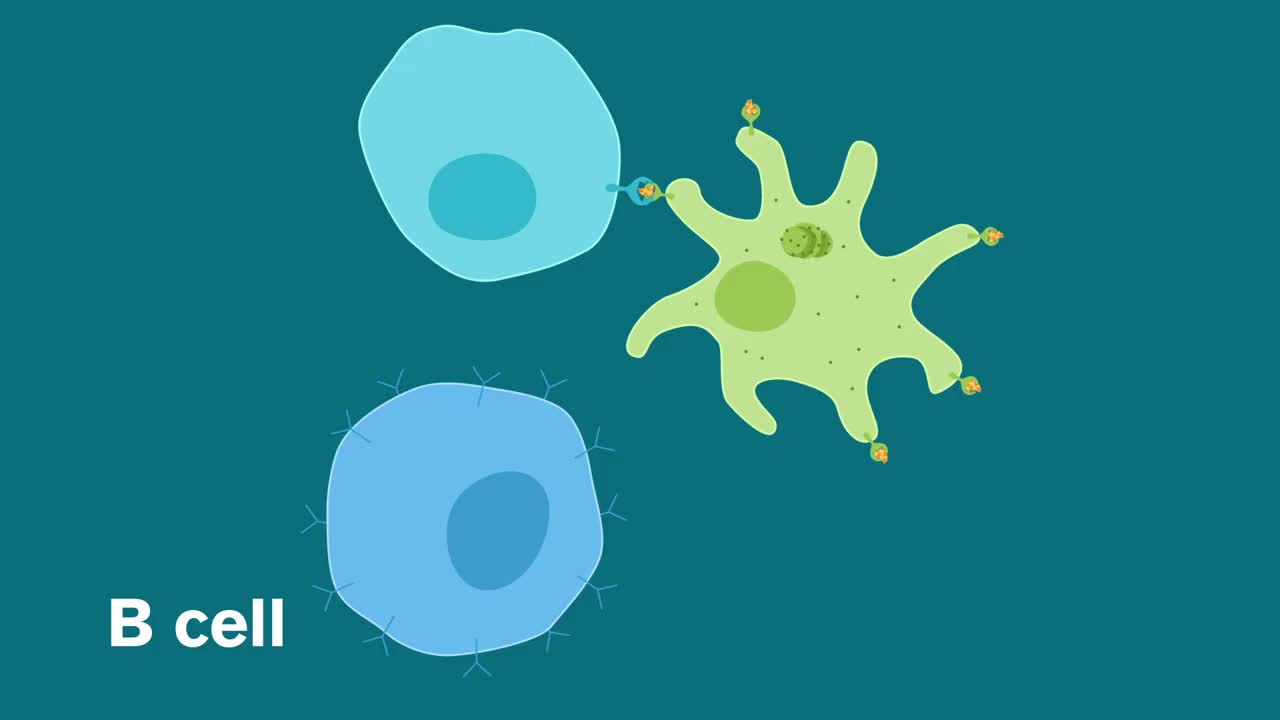
After we have been exposed to an infection, our immune system remembers the threat, in particular by producing antibodies. These are proteins that circulate in the blood and throughout the body; they quickly recognize and disable the invader upon contact, thereby preventing or minimizing illness. This is why we usually do not get sick with the same bug twice; we are immune. Vaccines mimic this process, encouraging the immune system to make antibodies without us having to go through the illness.
Some of the leading SARS-CoV-2 vaccine candidates are “mRNA vaccines,” based on incorporating the genetic blueprint for the key spike protein on the virus surface into a formula that when injected into humans instructs our own cells to make the spike protein. In turn, the body then makes antibodies against the spike protein and they protect us against viral infection.
This strategy is faster than more traditional approaches, which often involve generating weakened or inactivated forms of a live virus or making large amounts of the spike protein to determine whether they can prompt an antibody response.
Once a potential vaccine is discovered, a number of checkpoints exist before it can be administered to people. First are preclinical tests, which involve experiments in a laboratory and with animals. Scientists must ensure the vaccine candidate is not only effective, but also safe. For example, an antibody response to an imperfect vaccine could, under extremely rare circumstances, end up increasing the danger of becoming infected.
When the potential vaccine achieves the necessary preclinical results, clinical trials can begin in a small group of people. As the vaccine candidate advances, it is tested on increasing numbers of people, with scientists and doctors closely monitoring safety, efficacy and dosing. Upon successful completion of clinical trials, the vaccine candidate must be reviewed and approved by regulatory agencies such as the FDA before large-scale manufacturing and distribution gets underway and the licensed vaccine is administered widely.
-
 1:58
1:58
KIVI
3 years agoSome Idaho lawmakers against mandatory COVID-19 vaccines
3.35K7 -
 0:34
0:34
IOL-IndependentOnline
3 years ago $0.03 earnedWill the Covid-19 vaccines work against new variants of the virus?
4.95K -
 2:17
2:17
WTMJMilwaukee
3 years agoUW COVID-19 panel: Vaccines remain effective against known variants
80 -
 1:35
1:35
Mr Producer Media
3 years agoRand Paul: Fauci Is Going Against The Science Of Vaccines
1.52K9 -
 3:45
3:45
WXYZ
3 years agoMichigan's fight against COVID-19
3995 -
 LIVE
LIVE
Etheraeon
9 hours agoWorld of Warcraft: Classic | Fresh Level 1 Druid | 500 Follower Goal
415 watching -
 LIVE
LIVE
VapinGamers
1 hour ago🎮🔥Scrollin’ and Trollin’: ESO Adventures Unleashed!
196 watching -
 LIVE
LIVE
a12cat34dog
2 hours agoGETTING AFTERMATH COMPLETED :: Call of Duty: Black Ops 6 :: ZOMBIES CAMO GRIND w/Bubba {18+}
173 watching -
 LIVE
LIVE
NubesALot
5 hours ago $0.02 earnedDark Souls Remastered
117 watching -
 LIVE
LIVE
GamersErr0r
16 hours agoOverwatch 2
297 watching