Premium Only Content
Corona Virus Vaccines and introduction
Leading COVID-19 vaccine candidates rely on new technologies that have fast-tracked development and testing. Vaccines from Pfizer and Moderna have completed early phase 3 clinical trials and are reportedly under review at the US FDA for emergency use authorization (EUA) although safety surveillance continues. This video explains the principles underlying the leading DNA, messenger RNA (mRNA), and viral vector vaccine candidates, and how they might induce immunity to SARS-CoV-2 infection.
0:00 Introduction
1:21 Traditional vaccines
2:00 COVID-19 vaccine types in development
2:18 Making vaccines from a genetic sequence
2:45 Target antigen: the S protein
3:32 Genetic vaccines (DNA and mRNA)
4:18 Moderna/NIH and Pfizer/BioNTech vaccines
4:52 Viral vector vaccines
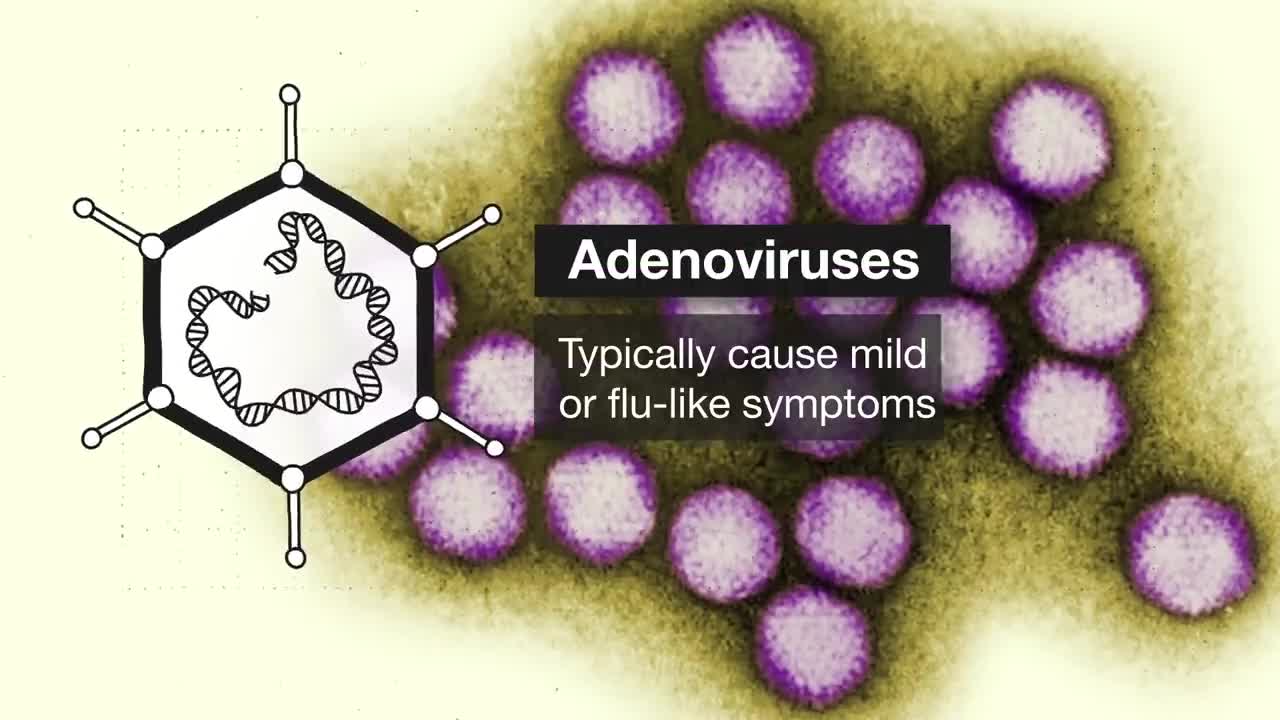
5:42 Adenovirus vectors (University of Oxford/AstraZeneca and Johnson & Johnson/Janssen Pharmaceuticals)
6:32 rVSV vector vaccine (Merck/IAVI)
7:15 Previous experience with next generation vaccines
7:50 Importance of Phase 3 Trials
-
 LIVE
LIVE
Badlands Media
9 hours agoBadlands Daily: June 4, 2025
471 watching -
 LIVE
LIVE
Wendy Bell Radio
5 hours agoMusk Blasts Beautiful Bill
4,671 watching -
 LIVE
LIVE
The Bubba Army
23 hours agoTrump Musk BreakUp? - Bubba the Love Sponge® Show | 6/04/25
1,638 watching -
 LIVE
LIVE
LFA TV
12 hours agoLFA TV ALL DAY STREAM - WEDNESDAY 6/4/25
1,996 watching -
 1:25:29
1:25:29
JULIE GREEN MINISTRIES
3 hours agoLIVE WITH JULIE
90.7K145 -
 1:40:14
1:40:14
Chicks On The Right
3 hours agoElon RIPS BBB, Hakeem still ranting about Project 25, & lack of pride merch is fascism
9.8K1 -
 1:39:17
1:39:17
Bitcoin on Rumble
1 hour agoBBB, Brrrr and Bitcoin
8.76K1 -
 LIVE
LIVE
AP4Liberty
2 hours ago $0.67 earnedElon Musk Calls Trump Megabill a ‘Disgusting Abomination’
274 watching -
 29:12
29:12
Producer Michael
20 hours agoTOURING A $13,750,000 LAS VEGAS MEGA MANSION WITH SPECTACULAR VIEWS OF THE STRIP!
39K8 -
 1:37:10
1:37:10
Nick Freitas
17 hours agoWhat We've Learned Through 26 Years of Marriage
24.3K2