Premium Only Content
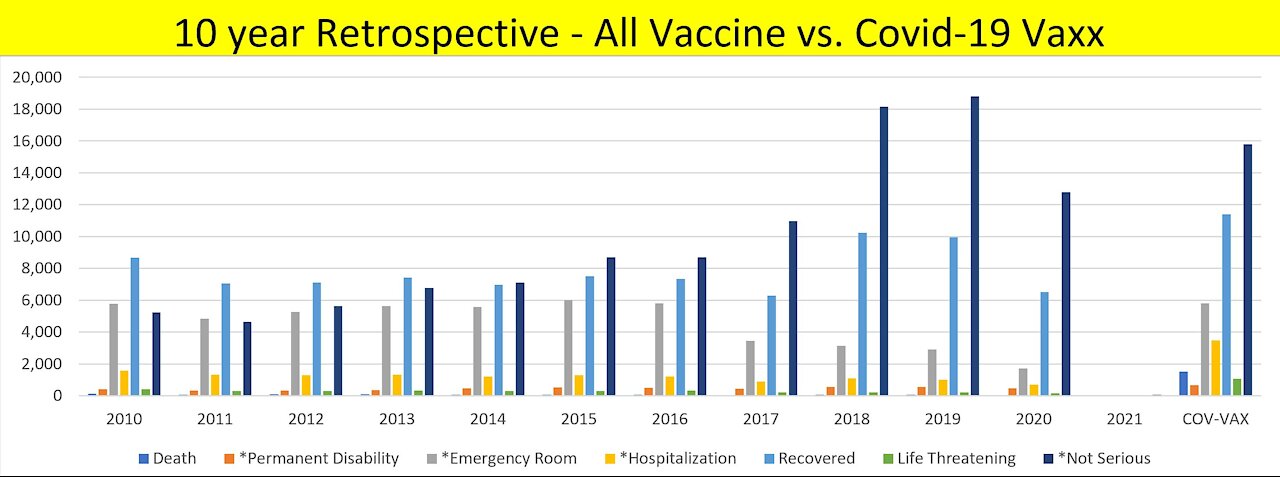
VAERS 10 year Adverse Effects retrospective versus the new unicorn vaxx
Take a look at how all other vaccines measure up against the new EXPERIMENTAL unicorn vaccines up to VAERS data dump on 3/12/2021
Follow me on:
Bitchute, Lbry, BrandNewTube, Vimeo, GAB, MINDS, MeWe, Minds, Twitter:
https://www.bitchute.com/channel/HEpBeuAz9n9P/
https://vimeo.com/511795894
https://brandnewtube.com/watch/vaers-data-up-to-2-4-2021-focus-of-fetal-demise-and-death_TcRFC6A3cG3XPn2.html
https://lbry.tv/@Welcometheeagle88:4/VAERS-data-up-to-2_4_2021---Focus-of-Fetal-Demise-and-Death:b
https://mewe.com/i/albertbenavides
https://gab.com/Welcometheeagle
https://www.minds.com/welcometheeagle88/
https://twitter.com/aba_3000
Please support me with one time donations or purchase of select dashboards:
Venmo: @Albert-Benavides-1
PayPal: BENAVIDEZ18
CashApp: $AlbertBenavides
This could be the problem. From ICAN: Informed Consent Action Network:
·
ICAN, through its attorneys, has written to HHS Acting Secretary Norris Cochran and Rochelle Walensky, the new Director of the Centers for Disease Control and Prevention, to demand that all adverse events following COVID-19 vaccination which are reported to the CDC’s new V-safe tool also be automatically reported to the Vaccine Adverse Events Reporting System (VAERS).
As discussed in prior legal updates, the issue of underreporting to VAERS has been highlighted for over 30 years and is still an ongoing problem, making reliable statistics regarding vaccine adverse reactions hard to come by. The CDC has now created a new smartphone-based tool used to track adverse events following COVID-19 vaccines called V-safe. At first glance, V-safe may seem like a welcome development: a modern tool that should easily allow vaccine recipients to report, and CDC to track, any adverse events experienced following a vaccination. However, there are serious potential problems created by V-safe.
For all intents and purposes, V-safe intercepts reports that would have otherwise been made by vaccine recipients into VAERS. Only if the CDC deems an adverse event reported to V-safe worthy will it be added to the VAERS database. This leaves a large universe of data regarding adverse events outside of VAERS and outside the public’s view.
When vaccinated for COVID-19, vaccine recipients are given a handout with information about what V-safe is, how it works, and how they can register for and use the program. V-safe is also explained in the FactSheets given out by both manufacturers at the time of vaccination. This “after vaccination health checker” uses text messages and web surveys to gather data about vaccine recipients following vaccination. Once enrolled, individuals will receive one text message per day for the first week after vaccination inquiring about how they are feeling. For the next five weeks, users will be sent one text message per week. The user responds to questions and prompts and, depending on the answers, may receive a call from someone at CDC to follow-up. If the CDC reaches out to a user and if the CDC feels that person’s adverse events rise to the level of “clinically important,” then a report may be submitted to VAERS.
This is unacceptable and is why ICAN has reached out to the acting director of the HHS and the new CDC Director to demand that all adverse events reported through V-safe are added to the VAERS database. This is absolutely necessary for complete and consistent data as well as for transparency as the public does not have access to any information exchanged through V-safe.
Especially where clinical trials for COVID-19 vaccines are not capturing all adverse reactions and the companies selling these products have no liability for injuries, the American public must be confident that safety surveillance is the highest priority with these vaccines. For this and the other reasons above, ICAN has demanded that all adverse events be systematically collected and tracked in one database that the public can access: VAERS.
ICAN will continue to take additional legal steps to hold HHS, FDA, and CDC accountable for vaccine safety. ICAN will never stop fighting for true informed consent and for transparency.
To receive more important legal updates, sign up for our newsletter at icandecide.org.
-
 1:08
1:08
Newsy
4 years agoDose Of Truth: Will The COVID Vaccine Cause Adverse Effects?
2.45K -
 0:19
0:19
bobbygoss30
4 years agoUnicorn 🦄 sounds
59 -
 5:14
5:14
Electron Alley
4 years ago $0.01 earnedErie Effects: A Year in the Life of a Lake, 2011 - North
40 -
 5:16
5:16
Electron Alley
4 years ago $0.01 earnedErie Effects: A Year in the Life of a Lake, 2011 - West
453 -
 1:07:26
1:07:26
Inverted World Live
14 hours agoThe War Against Robots w/ Joe Allen
104K5 -
 6:08:31
6:08:31
SpartakusLIVE
13 hours agoWARZONE NUKE IS BACK?! || Solo Challenge CHAMPION to start, duos w/ the Dawg later
107K1 -
 1:00:18
1:00:18
Man in America
15 hours agoBig Pharma’s Empire of Lies Is COLLAPSING as People Turn to Natural Medicine
68.3K27 -
 7:17:44
7:17:44
Drew Hernandez
17 hours agoGHISLAINE MAXWELL SAYS CLAIMS EPSTEIN WAS INTELLIGENCE ASSET ARE BULLSH*T?!
39.8K41 -
 29:54
29:54
Afshin Rattansi's Going Underground
1 day agoUkraine: Prof. Anatol Lieven SLAMS Europe’s ‘BLOODY STUPIDITY’ as Trump Negotiates with Putin
36K8 -
 15:27
15:27
robbijan
1 day ago $2.48 earnedThe Emperor’s New Labubu & The Spiritual War Behind Everything
56.3K45