Premium Only Content

DNA Vaccine by AstraZeneca - READ the LABEL - DEFINE EACH WORD
HEALTH
VERIFY: What is AstraZeneca's vaccine, how did it perform in trials?
The drug company gave out its vaccine two different ways in their trials. One way proved to be noticeably more effective.
Author: Matt Gregory
Published: 6:14 AM EST November 23, 2020
Updated: 6:16 AM EST November 23, 2020
Facebook Twitter
WASHINGTON — Astra Zeneca reports its coronavirus vaccine is "highly effective" at preventing COVID-19.
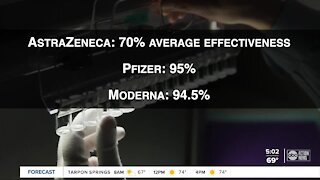
According to the drug maker, its vaccine had an effectiveness of 70%, an average of two different dosage regimens.
As more vaccines release results of late-stage trials, you are going to get more numbers you may not understand thrown at you. That’s why the Verify team is here.
We take the study results to vetted experts to explain them.
Question:
What is AstraZeneca’s vaccine and what did the trials show?
Answer:
It is a “vector virus” vaccine and the trials showed a 90% effectiveness and 62% effectiveness; resulting in an overall 70% efficacy.
Our Sources:
The drug company AstraZeneca and Johns Hopkins University Vaccine expert Dr. William Moss.
Our Process:
This is going to read a lot like a biology class, so let’s break it down.
AstraZeneca’s vaccine is a different type of vaccine than the Pfizer and Moderna vaccines. The first two directly injected the coronavirus’ genetic code into the patients to produce an immune response.
“[AstraZeneca] instead of injecting the nucleic acid an RNA or DNA version of that gene, the gene is delivered in another virus,” Dr. Moss explained.
Here is how the company’s “vector virus” vaccine works:
Researchers put the genetic code of coronavirus into a dead or weakened Adenovirus. Then, injected that virus into patients to deliver the genetic code to the body. Then the body can create an immune response to coronavirus.
“They are just different strategies to trick our own bodies to make the virus protein release it in our bodies and [the] immune system responds to it,” Dr. Moss explained.
How did AstraZeneca’s trials go?
AstraZeneca did two different dosages in their studies. In one trial, they gave patients a half dose and then a month later a full dose that proved to have an efficacy rating of 90%.
The other trial gave patients two full doses a month apart and that proved to have a 60% efficacy rating.
The results of the combined studies averaged a 70% efficacy rating.
Other notes from the study:
AstraZeneca’s vaccine can be stored at refrigerator temperatures. Whereas, Pfizer’s vaccine required extreme cold temperatures.
AstraZeneca also reported their vaccine had a strong response in the elderly. The older population is one of COVID-19’s most at-risk age groups.
AstraZeneca reports that it will soon apply for emergency use authorization through the WHO.
-
 0:40
0:40
Newsy
4 years agoAstraZeneca: Vaccine Averaged 70% Effectiveness
1.21K2 -
 0:22
0:22
Newsy
5 years agoAstraZeneca COVID-19 Vaccine Trial Participant Dies
2.94K2 -
 0:59
0:59
Newsy
5 years agoAstraZeneca Pauses COVID-19 Vaccine Trial
11.2K1 -
 1:49
1:49
WPTV
4 years agoSouth Florida doctor optimistic about AstraZeneca COVID-19 vaccine
714 -
 0:30
0:30
Newsy
5 years agoAstrazeneca Resumes Phase 3 Coronavirus Vaccine Trial
1.49K4 -
 0:59
0:59
Newsy
4 years agoAstraZeneca Admits To Mistakes In Coronavirus Vaccine Trial
2.15K1 -
 1:20
1:20
Newsy
5 years agoFDA Widens Investigation Into AstraZeneca COVID-19 Vaccine
7691 -
 0:54
0:54
Newsy
4 years agoUnited Kingdom Starts Accelerated Review Of AstraZeneca Vaccine
1.59K -
 0:21
0:21
WFTS
4 years agoAstraZeneca, Oxford say their vaccine is up to 90% effective
102 -
 0:37
0:37
Newsy
4 years agoBloomberg: AstraZeneca Wants To Hold Another Vaccine Trial
1.69K