Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
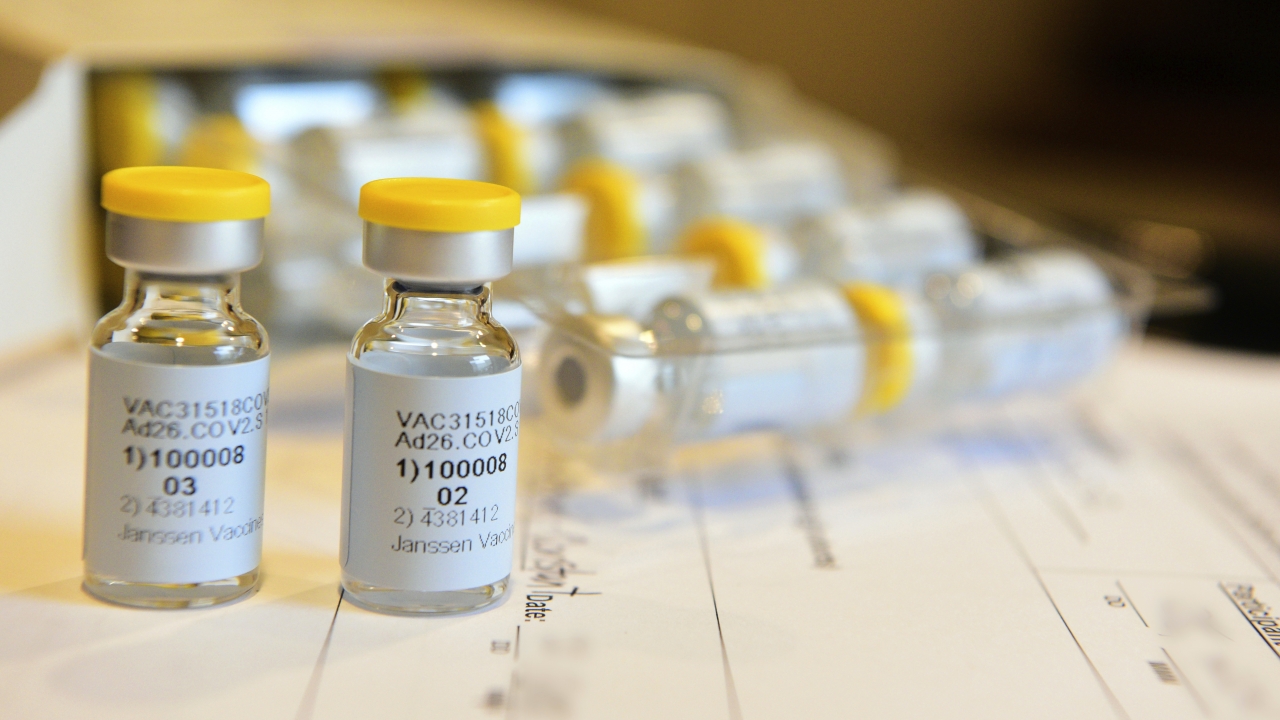
Johnson & Johnson Pauses Vaccine Study After Unexplained Illness
4 years ago
1.45K
Sci/Health
Coronavirus
U.S.
covid-19
coornavirus
COVID
vaccine
Johnson & Johnson
health news
unexplained illness
investigating
Johnson & Johnson is investigating after one of the participants in its study on a potential coronavirus vaccine became ill.
Loading 3 comments...
-
 3:20
3:20
Newsy
2 years agoRussia suspends only remaining major nuclear treaty with US
8.1K14 -
 3:44
3:44
WXYZ
4 years agoAsk Dr. Nandi: AstraZeneca pauses coronavirus vaccine trial after unexplained illness in volunteer
132 -
 1:26
1:26
WFTS
4 years agoAstraZeneca COVID-19 vaccine trial paused after one illness
1.21K1 -
 0:43
0:43
Newsy
4 years agoJohnson & Johnson Begins Phase 3 COVID-19 Vaccine Trials
1.28K2 -
 1:21
1:21
Newsy
5 years agoJohnson & Johnson Committing To Coronavirus Vaccine
470 -
 0:23
0:23
KTNV
4 years agoAstraZenca pauses coronavirus vaccine trials
6531 -
 0:59
0:59
Newsy
4 years agoAstraZeneca Pauses COVID-19 Vaccine Trial
11.2K1 -
 3:06
3:06
JoyFull Soul with Imara
4 years agoStudy after 45...Yikes!!! :)
10 -
 3:41
3:41
WXYZ
4 years agoCOVID-19 vaccine round-up: Johnson & Johnson begins Phase 3 trials in the United States
275 -
 2:41
2:41
Newsy
4 years agoAstraZeneca's COVID-19 Vaccine On Hold After Sickness
1.55K5