Premium Only Content
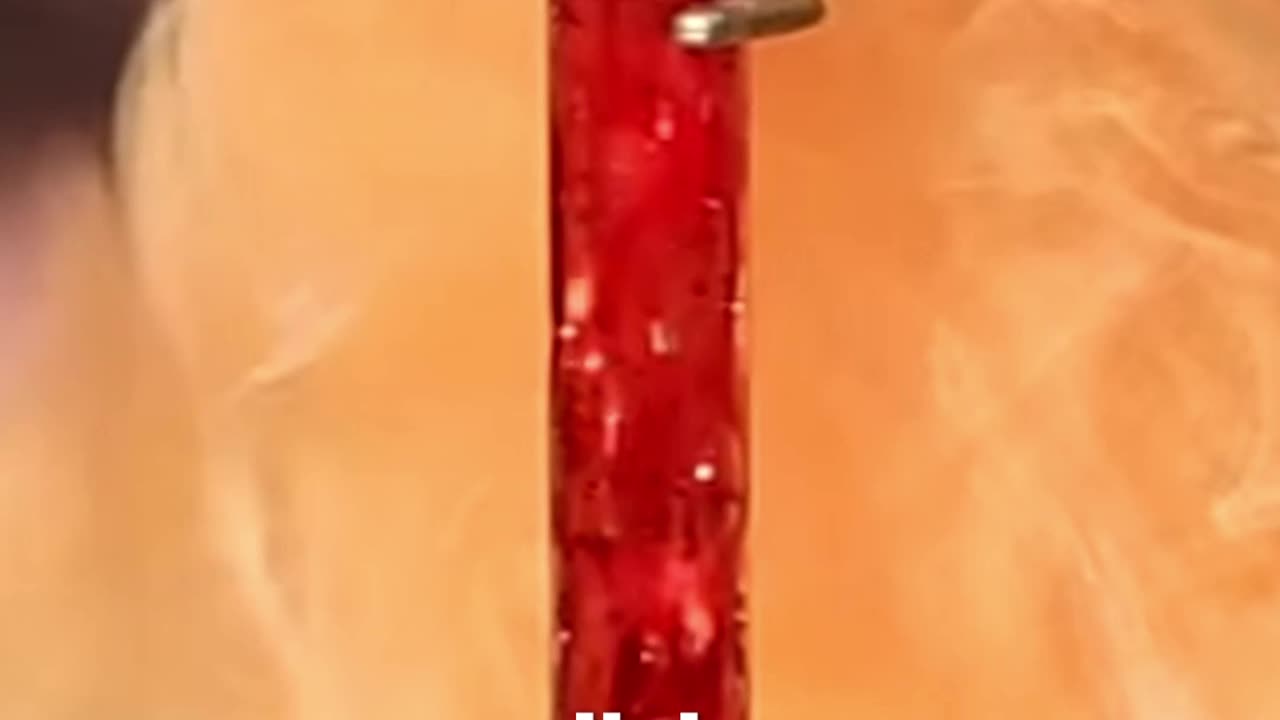
Bromine vs Aluminum ⚡ From Foil to Plastics and Dyes #chemistry #science
#chemistry #science #shorts #curious
This demo shows liquid bromine reacting violently with aluminum foil to form aluminum bromide. The halogen oxidizes aluminum, releasing heat and fumes in a striking redox reaction. Aluminum bromide is a Lewis acid catalyst used in Friedel–Crafts chemistry to build aromatic compounds that end up in plastic packaging, synthetic dyes for fabrics and inks, and pharmaceutical ingredients.
Safety note: Bromine is highly toxic and corrosive; this experiment should only be performed in a controlled laboratory with proper PPE and ventilation.
Attribution: Clip source: NileRed — original upload Reacting Bromine and Aluminum (2015) (Archive.org mirror of YouTube). Transformed under fair use: narration, music, trim (~30s), reframed visuals, educational subtitles.
https://archive.org/details/nilered-youtube-archive
-
 0:34
0:34
Fantasia Fables
7 hours agoShackleton’s Men on the Floes #Antarctica #Survival
14 -
 2:04:16
2:04:16
Inverted World Live
9 hours agoTwo Texas Men Plotted to Invade Haiti | Ep. 146
64.3K7 -
 2:54:20
2:54:20
TimcastIRL
7 hours agoClinton Judge JUST DISMISSED James, Comey Indictment, Trump DOJ APPEALS | Timcast IRL
224K133 -
 3:01:32
3:01:32
SpartakusLIVE
5 hours agoCreator House LIVE STREAM || ASSUAGING the RAGE of viewers by streaming DEEP into the Night
29.4K1 -
 17:37
17:37
MetatronHistory
1 day agoThe REAL Origins of the GREEKS
26.8K12 -
 1:19:32
1:19:32
The Daily Signal
9 hours ago $11.79 earned🚨BREAKING: James Comey & Letitia James NOT "Off the Hook" on Indictments, Sen. Kelly Court Martial?
32.2K4 -
 4:46:23
4:46:23
Drew Hernandez
1 day agoBONDI DOJ BLOWS IT ON COMEY/LETICIA INDICTMENTS?!
42.2K21 -
 2:34:03
2:34:03
PandaSub2000
4 days agoLIVE 10:30pm ET | CARMEN SANDIEGO
35.6K1 -
 12:10
12:10
Robbi On The Record
1 day ago $5.49 earnedKarmic Disclosure and Predictive Programming
38K5 -
 4:57
4:57
Gamazda
7 hours ago $6.86 earnedAerosmith - Dream On (Piano by Gamazda)
25.2K14