Premium Only Content
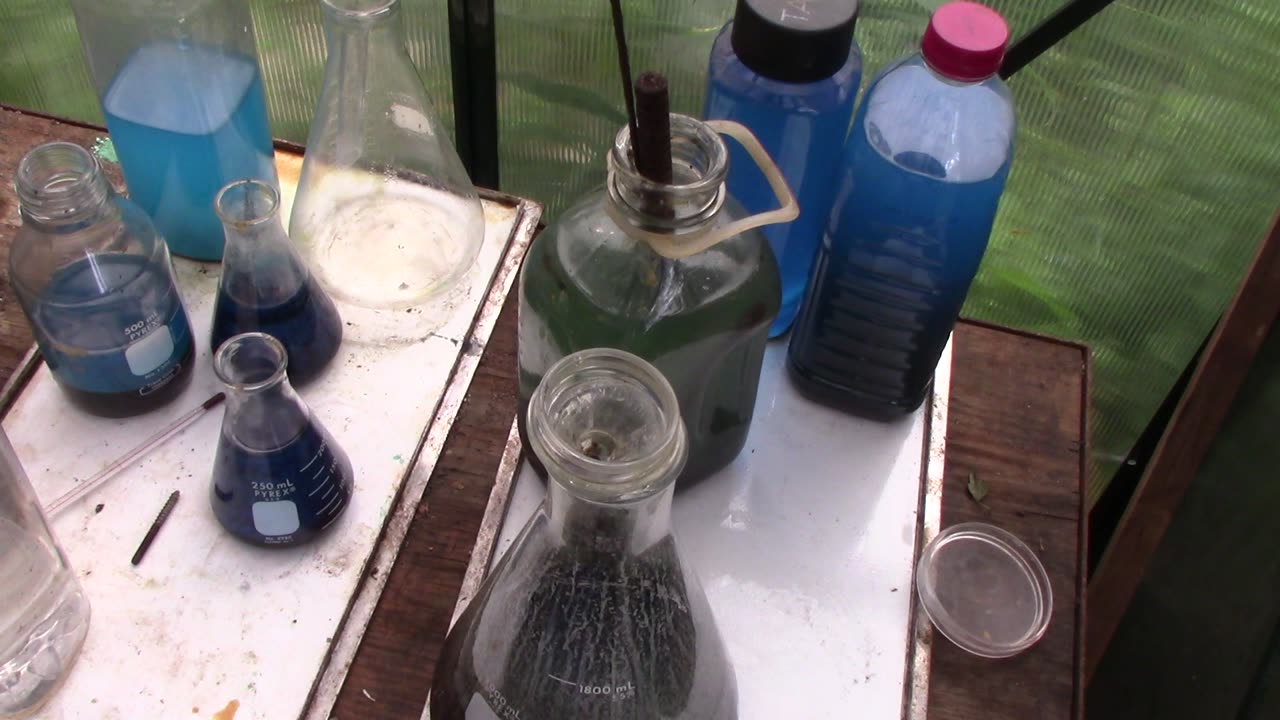
Results of low-grade pin base metal removal with sulfuric acid
This was a test to see how well concentrated sulfuric acid heated to a roughly water's boiling point (212F/100C) for several hours, using low volumes of acid with several changes over time, would remove base metals and reduce corrosive gas emissions, giving a waste solution of easy to treat sulfate salts. Turns out it worked incredibly well! A few hours for each boil, a day of soaking the cooled solids in water to turn the solid grey Cu(I) sulfate to aqueous blue Cu(II) sulfate, pour off the solution and repeat until only a few pin remnants and brown 'mud' remains. About 3 days, and the base metal is mostly gone. The mud and small fragments of pins can then be cleaned up with HCl, much much less HCl than would be required to remove all the base metals from the start. And vastly cheaper than using nitric, without the risk of big blobs of metastannic acid goo formation. Also no risk of accidentlaly dissolving gold, since combining trace sulfuric acid with HCl will NOT dissolve gold. Gas formation is fairly low, and a modest breeze outside easily takes care of what SO2 gas that is released. The only difficult part is filtering the brown sediment 'mud' which holds most of the gold. The repeated acid boils break up the thin foils into very fine particles so you do need a good vacuum filter setup to catch it all in a reasonable time frame. Settling and gravity filtering is just too slow.
-
 19:01
19:01
Stephen Gardner
9 hours agoAlex Jones: ‘Trump Is About to Do Something MASSIVE!'
27.9K103 -
 LIVE
LIVE
Drew Hernandez
22 hours agoCANDACE OWENS ACCEPTS TPUSA INVITATION TO DISCUSS SUSPICION BEHIND CHARLIE KIRK ASSASSINATION?!
1,238 watching -
 2:52:10
2:52:10
TimcastIRL
5 hours agoDrunk Raccoon Becomes Top US Story After Getting Plastered, Passing Out In Bathroom | Timcast IRL
210K82 -
 LIVE
LIVE
SpartakusLIVE
8 hours agoRule #1 - The BEST Loot is ALWAYS in the OTHER GUY'S BAG
635 watching -
 2:16:27
2:16:27
ThatStarWarsGirl
5 hours agoTSWG LIVE: CHRISTMAS IS COMING! Stargate's BACK & DC is Doomed?!
16.9K3 -
 2:38:16
2:38:16
I_Came_With_Fire_Podcast
12 hours agoDid Pete Hegseth Commit WAR CRIMES | No more INCOME TAX | More Fraud in Minnesota W/ Mike Caldarisie
20K10 -
 1:32:30
1:32:30
Adam Does Movies
7 hours ago $0.55 earnedTalking Movies + Ask Me Anything - LIVE
16.5K1 -
 2:12:14
2:12:14
TheSaltyCracker
5 hours agoWar Crimes ReeEEStream 12-03-25
72.1K156 -
 1:31:59
1:31:59
Glenn Greenwald
6 hours agoTrump Administration Claims to Save Hundreds of Millions of Lives by Blowing Up Drug Boats; Ethan Klein's Unhinged Vengeance & Lawsuits Against Other YouTubers: With Taylor Lorenz | SYSTEM UPDATE #553
104K134 -
 19:14
19:14
MetatronCore
22 hours agoHow Propaganda works on your Brain
16.4K6