Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
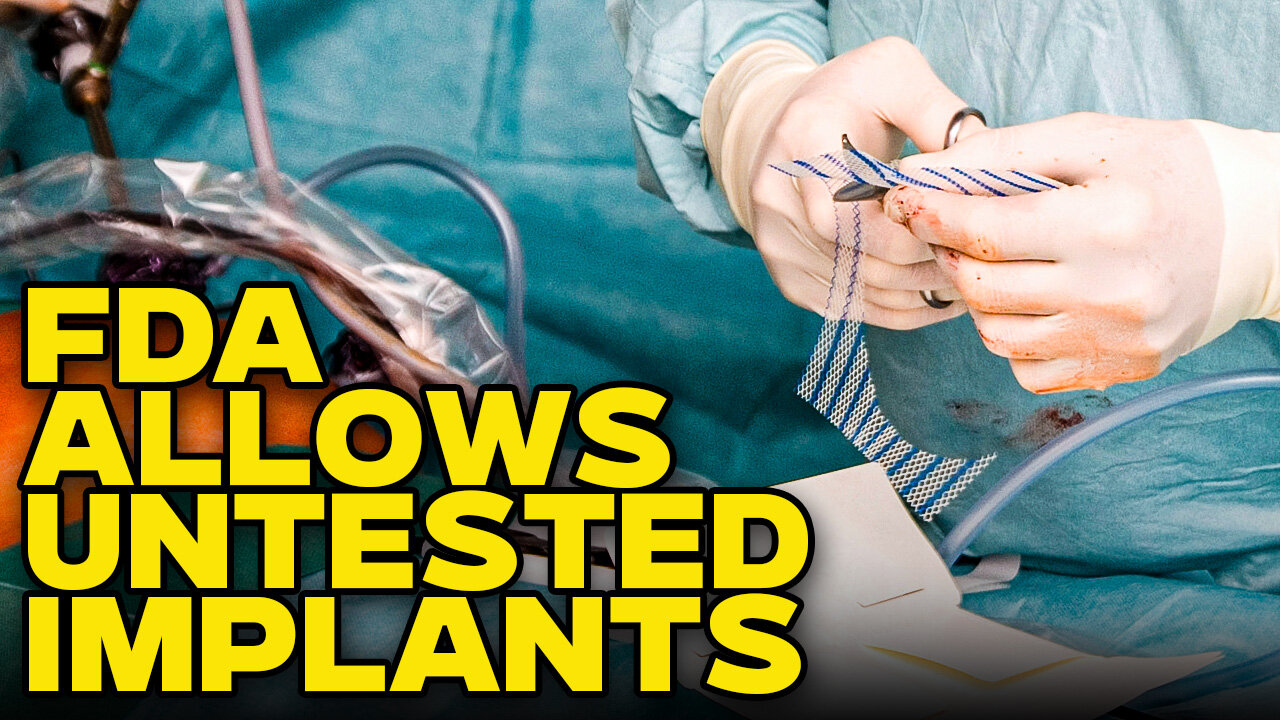
FDA Is Allowing Big Pharma To Use 70's Data To Pass Inspections
1 month ago
4
Medical devices are like the wild west. in order to get onto the market, the manufacturer just has to show that that device is substantially equivalent to a device that's already on the market. What's disturbing about it is that these manufacturers can piggyback off of devices that entered the market before 1976 and that predicate device can be a device that was recalled from the market. Caleb Cunningham is joined by Kelsey Stokes, a medical device attorney with the law firm of Fleming, Nolan and Jez, to explain more.
Loading 1 comment...
-
 5:25
5:25
America's Lawyer
5 days agoTrump Lays Plan To Steal Jewels From The Smithsonian Museum
21 -
 LIVE
LIVE
Drew Hernandez
10 hours agoEPSTEIN VICTIMS SPEAK OUT & TRUMP DOUBLES DOWN
958 watching -
 1:36:41
1:36:41
FreshandFit
6 hours agoWe Are QUITTING YouTube...
51.9K33 -
 2:34:22
2:34:22
TheSaltyCracker
6 hours agoDrug Smugglers Blown Up 9-03-25
93.3K190 -
 3:12:59
3:12:59
VapinGamers
5 hours ago $1.05 earnedGrim Trials - Game Review/Playthru - Rougelight Dungeon Crawler - !rumbot !music
26.7K -
 2:47:55
2:47:55
Mally_Mouse
13 hours ago🎮 Let's Play!! -- Jak 2 pt. 16
50.9K2 -
 52:23
52:23
MattMorseTV
7 hours ago $16.87 earned🔴The Cartels are SCREWED.🔴
114K130 -
 1:32:46
1:32:46
Badlands Media
21 hours agoAltered State S3 Ep. 44: Epstein Files, Corrupt Judges, and the College Grift
54K2 -
 21:09
21:09
Bearing
18 hours agoAustralian “Racist” Protest EXPLODES 💥 Glowies, Brawls & Media Spin 📣
28.9K37 -
 2:53:23
2:53:23
Tundra Tactical
6 hours ago $2.86 earnedTwo Vets, One Ouija Board, Zero Good Decisions
25.1K1