Premium Only Content
Differential Equations: Potassium-40 Decay: The chemical element potassium is a soft metal that can
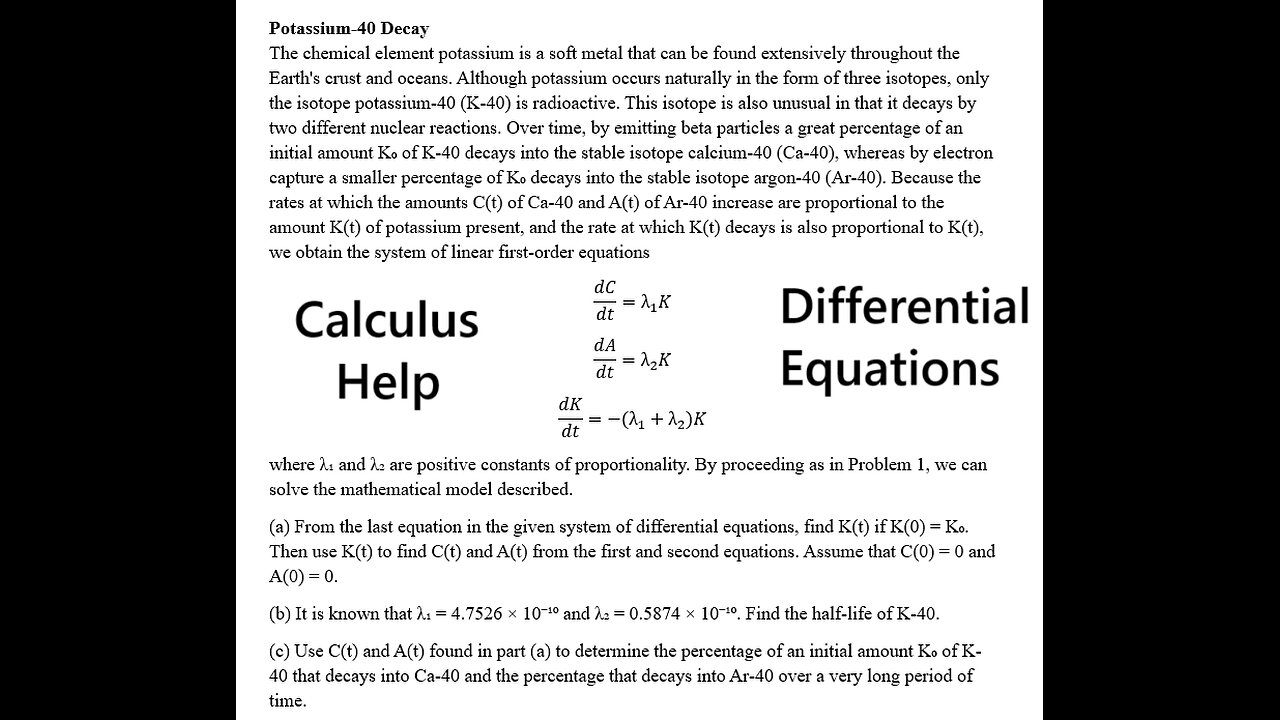
Potassium-40 Decay
The chemical element potassium is a soft metal that can be found extensively throughout the Earth's crust and oceans. Although potassium occurs naturally in the form of three isotopes, only the isotope potassium-40 (K-40) is radioactive. This isotope is also unusual in that it decays by two different nuclear reactions. Over time, by emitting beta particles a great percentage of an initial amount K₀ of K-40 decays into the stable isotope calcium-40 (Ca-40), whereas by electron capture a smaller percentage of K₀ decays into the stable isotope argon-40 (Ar-40). Because the rates at which the amounts C(t) of Ca-40 and A(t) of Ar-40 increase are proportional to the amount K(t) of potassium present, and the rate at which K(t) decays is also proportional to K(t), we obtain the system of linear first-order equations
dC/dt=λ_1 K
dA/dt=λ_2 K
dK/dt=-(λ_1+λ_2)K
where λ₁ and λ₂ are positive constants of proportionality. By proceeding as in Problem 1, we can solve the mathematical model described.
(a) From the last equation in the given system of differential equations, find K(t) if K(0) = K₀. Then use K(t) to find C(t) and A(t) from the first and second equations. Assume that C(0) = 0 and A(0) = 0.
(b) It is known that λ₁ = 4.7526 × 10⁻¹⁰ and λ₂ = 0.5874 × 10⁻¹⁰. Find the half-life of K-40.
(c) Use C(t) and A(t) found in part (a) to determine the percentage of an initial amount K₀ of K-40 that decays into Ca-40 and the percentage that decays into Ar-40 over a very long period of time.
#CalculusHelp
#DiffernetialEquations
#SystemOfEquations
-
 LIVE
LIVE
Kim Iversen
1 hour agoZelensky BLINDSIDED By Trump In White House Meeting | UK Bans RACIST JEWS From Attending Soccer Match
6,645 watching -
 LIVE
LIVE
The Jimmy Dore Show
39 minutes agoJohn Bolton Staring At LIFE IN PRISON! Kash Patel Pushes PURE BS Line About Kirk Assassination!
3,885 watching -
 LIVE
LIVE
Nerdrotic
2 hours agoRacist Academics Attack Tolkien | Hollywood to Strike AGAIN? | AI Doomsday - Friday Night Tights 376
5,236 watching -
 LIVE
LIVE
Dr Disrespect
8 hours ago🔴LIVE - DR DISRESPECT - ARC RAIDERS - THE ULTRA EXTRACTION GAME
1,074 watching -
 27:49
27:49
Robbi On The Record
19 hours ago $0.14 earnedRevelation, the End Times, and Satan’s Little Season part II - ft JT
2524 -
 54:37
54:37
HotZone
4 days agoTen Hostages Missing! Will Hamas Keep Its Word?
8.43K2 -
 8:05
8:05
Rethinking the Dollar
6 hours agoFiat’s Endgame? Gold & Silver Lines Don't Lie
2244 -
 LIVE
LIVE
LFA TV
21 hours agoLIVE & BREAKING NEWS! | FRIDAY 10/17/25
1,295 watching -
 1:13:16
1:13:16
vivafrei
3 hours agoJohn Bolton is a DUMB CRIMINAL (Allegedly) - Trans Madness in Loudoun Country! Tampon Tim AND MORE!
63.1K26 -
 2:45:30
2:45:30
Barry Cunningham
16 hours agoBREAKING NEWS! PRESIDENT TRUMP MEETS WITH UKRAINE PRESIDENT ZELENSKY!
48.7K16