Premium Only Content
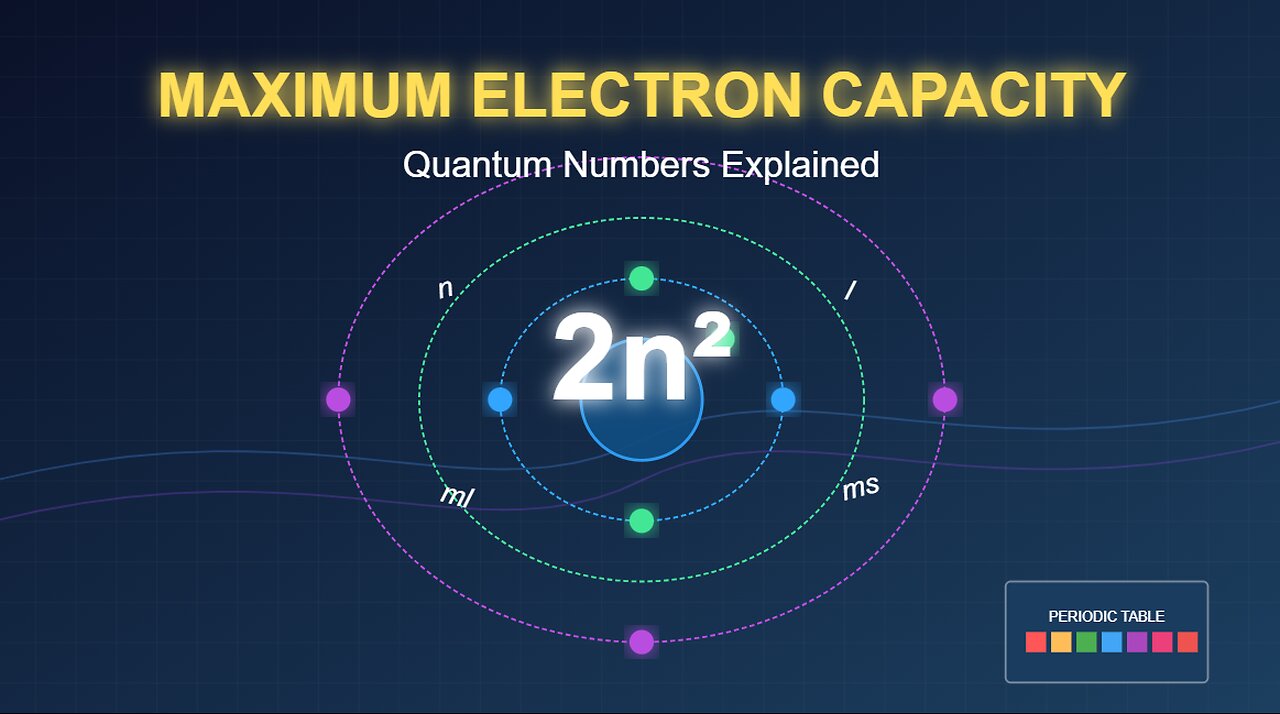
Quantum Numbers: How to Calculate Maximum Electron Capacity (2n² Formula Explained)
Struggling with electron configurations? This 3:40 video breaks down how quantum mechanics determines exactly how many electrons can fit in each shell and subshell.
Learn how the four quantum numbers (n, l, ml, ms) and the Pauli Exclusion Principle work together to create the pattern of electron distribution across atoms. We'll derive the essential formulas:
• Single orbital: 2 electrons
• Subshells: 2(2l+1) electrons
• Main shells: 2n² electrons
Perfect for AP Chemistry, college General Chemistry, and Physical Chemistry students. This concise explanation connects quantum theory to the periodic table's structure and helps you understand why electron configurations follow their specific patterns.
#QuantumNumbers #ElectronConfiguration #ChemistryTutorial #APChemistry #OrganicChemistry #QuantumMechanics #ChemistryHelp
Keywords: electron configuration, quantum numbers, Pauli exclusion principle, 2n2 formula, chemistry tutorial, electron shells, subshells, orbitals, maximum electron capacity, periodic table, quantum mechanics basics
-

Side Scrollers Podcast
1 day ago🔴SIDE SCROLLERS SUB-A-THON🔴FINAL DAY!🔴Craig Makeover + US Dart Throw + More!
351K27 -
 2:05:58
2:05:58
TimcastIRL
7 hours agoSHOTS FIRED, Leftists ATTACK Coast Guard & Feds In SHOCK Terror Attack | Timcast IRL
232K131 -
 1:07:25
1:07:25
Man in America
13 hours agoThe BRICS War on the Dollar Just Hit Endgame—What's Next Changes EVERYTHING
20.3K12 -
 3:23:45
3:23:45
SOLTEKGG
4 hours ago🔴LIVE - Community Game Night - GIVEAWAY
24K2 -
 LIVE
LIVE
SpartakusLIVE
7 hours ago#1 Friday Night HYPE, viewers GLUED to the screen
354 watching -
 55:50
55:50
NAG Podcast
5 hours agoAda Lluch: BOLDTALK W/Angela Belcamino
8.73K1 -
 LIVE
LIVE
VapinGamers
2 hours agoKellan Graves - Fallen - Game Review and Game KeyGiveaway - !rumbot !music
168 watching -
 1:06:41
1:06:41
MattMorseTV
5 hours ago $0.16 earned🔴Trump PREPARES for WAR with VENEZUELA.🔴
36.2K63 -
 39:59
39:59
Clownfish TV
9 hours agoHollywood NO MORE! Animation Industry Will DIE First?! | Clownfish TV
8.16K2 -
 25:57
25:57
The Kevin Trudeau Show Limitless
2 days agoThe Sound Of Control: This Is How They Program You
61.2K19