Premium Only Content
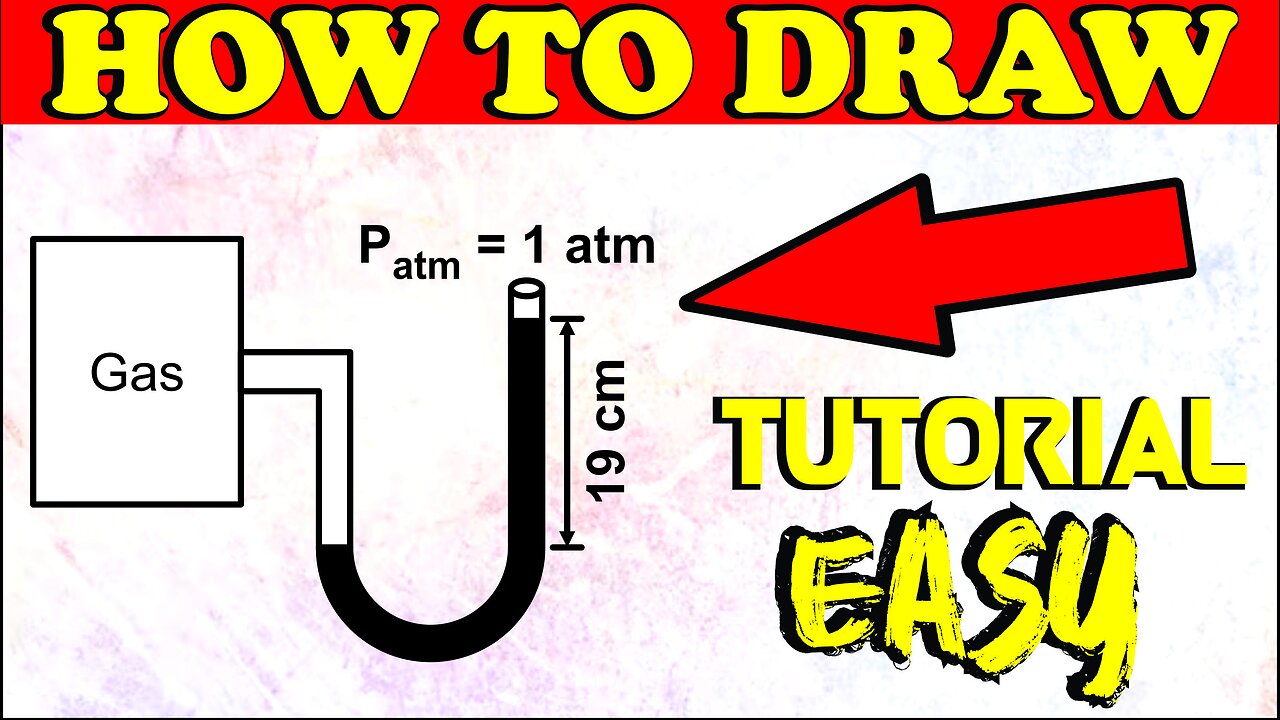
A gas Ax having mass of 100g is confined in a container of volume 16L By Seekh Raha Hoon
A gas Ax having mass of 100g is confined in a container of volume 16L maintained at 300K and a manometer is attached as shown, By Seekh Raha Hoon
A gas Ax having mass of 100 g is confined in a container of volume 16 L maintained at 300 K and a manometer is attached as shown, value of x is: (R = 0.08 atm -L/mole-K) (Atomic mass of A = 24)
Step by step video, text & image solution for A gas A_(x) having mass of 100g is confined in a container of volume 16L maintained at 300K and a manometer is attached as shown, value of x is: (R=0.08 atm-L/mole-K) (Atomic mass of A=24) by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Search Query
Calculate the number of moles of gas present in mcq
Calculate the number of moles of gas present in class 11
Calculate the number of moles of gas present in chemistry
A rigid vessel of volume 0.50 m3
50 ml of a gaseous mixture of CO and CH4
A 10 litre container consist of 1 mole
A gas is filled in the glass bulb of capacity 10 litre
-
 1:55
1:55
Dr Disrespect
2 days agoPeak Focus. No Crash. This Is KENETIK
3.62K7 -
 2:06:36
2:06:36
Side Scrollers Podcast
16 hours agoThis is the Dumbest Story We’ve Ever Covered… | Side Scrollers
62.8K15 -
 15:37
15:37
The Pascal Show
15 hours ago $0.03 earnedCANDACE OWENS DISAPPEARS?! Candace Owens Goes Into Hiding After Revealing A**assination Claims
1.45K -
 18:05
18:05
GritsGG
1 day agoThis Duo Lobby Got a Little Spicy! We Have Over 20,000 Wins Combined!
18.5K -
 1:12:29
1:12:29
PandaSub2000
3 days agoSonic Galactic | GAME ON...ly! (Edited Replay)
14.3K3 -
 LIVE
LIVE
Lofi Girl
3 years agolofi hip hop radio 📚 - beats to relax/study to
193 watching -
 21:23
21:23
Neil McCoy-Ward
16 hours ago🚨 While You Were Distracted TODAY... (This Quietly Happened!!!)
9.2K7 -
 6:09:42
6:09:42
SpartakusLIVE
7 hours agoLIVE from OCEAN FRONT || ENERGIZED Wins and TOXIC Comms
255K14 -
 1:35:45
1:35:45
Tucker Carlson
8 hours agoTucker Puts Piers Morgan’s Views on Free Speech to the Ultimate Test
55.3K255 -
 2:06:16
2:06:16
TheSaltyCracker
8 hours agoMedia Justifies Attack on National Guard ReeEEStream 11-26-25
115K276