Premium Only Content
Regulatory Affairs - Applications of QSR for Medical Devices: 21 CFR Part 820 and ISO 13485 by Peivand Pirouzi, Ph.D.
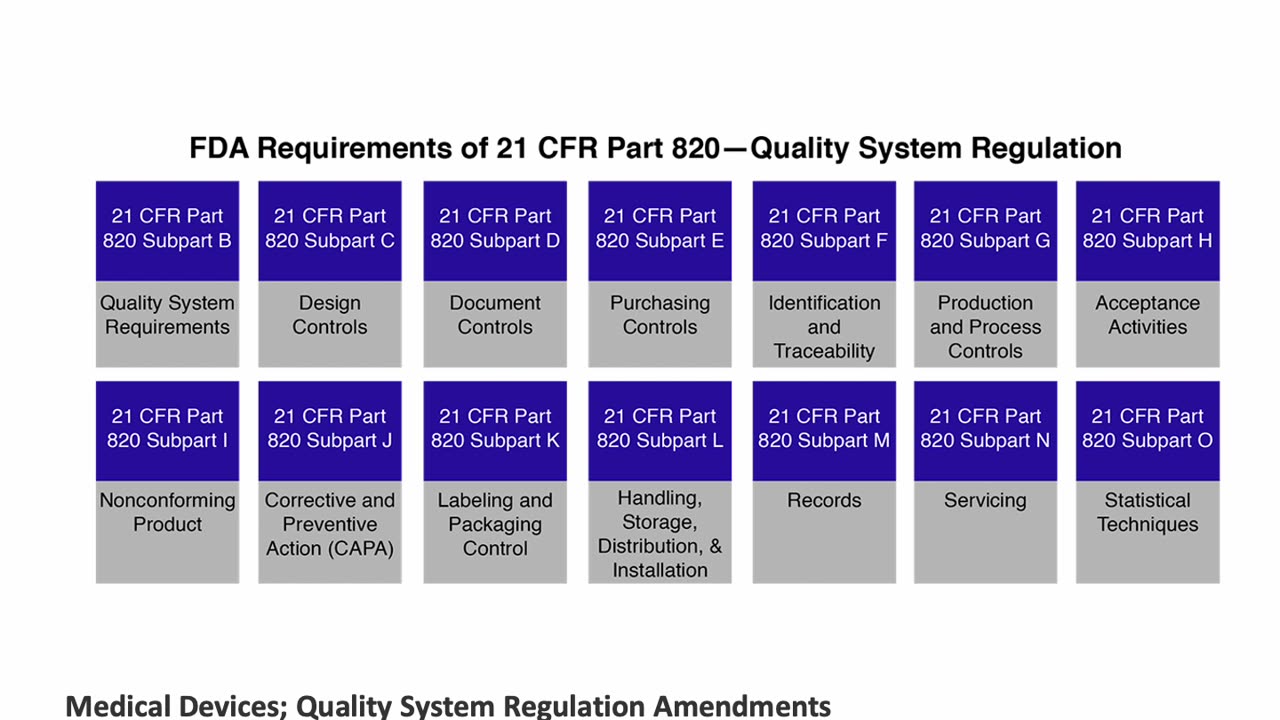
Explore the practical applications of Quality System Regulation (QSR) for medical devices in this insightful presentation by Peivand Pirouzi, Ph.D. This video focuses on the requirements of 21 CFR Part 820, the FDA’s framework for medical device quality systems, and ISO 13485, the global standard for quality management in the medical device industry. Special attention is given to the harmonization of 21 CFR Part 820 with ISO 13485, simplifying compliance for organizations operating in both domestic and international markets.
Professor Pirouzi provides an in-depth analysis of these regulations, offering guidance on implementation strategies to enhance compliance, product safety, and efficiency. Whether you’re navigating domestic requirements or aiming for corporate-level online training and certification by Crown College of Canada (www.crowncollege.ca), this session equips you with the knowledge to succeed.
-
 1:09:09
1:09:09
Omar Elattar
8 hours agoThe Brain Experts: Your Brain Can Rewire Itself At Any Age & Here's How!
22.5K4 -
 4:30:37
4:30:37
IcyFPS
5 hours agoLIVE - Wuchang Fallen Feathers x Borderlands w/ pope!
29.4K2 -
 29:24
29:24
Afshin Rattansi's Going Underground
18 hours agoWas Epstein a Mossad Agent? Will Obama go to Prison? (Afshin Rattansi vs Alan Dershowitz)
30.8K22 -
 4:26:54
4:26:54
Nerdrotic
11 hours ago $34.41 earnedFantastic Four Baby Steps V Superman's James Gunn, South Park Returns | Friday Night Tights 364
114K10 -
 3:17:40
3:17:40
megimu32
5 hours agoOFF THE SUBJECT: FAFO Friday! Cops, Crash, Kombat & Chaos!
25.9K6 -
 10:17:28
10:17:28
GrimmHollywood
14 hours ago🔴LIVE • GRIMM HOLLYWOOD • CLIP FARMING 101 •
21.4K1 -
 1:07:56
1:07:56
Glenn Greenwald
11 hours agoIsrael-Made Famine Crisis Finally Recognized | SYSTEM UPDATE #493
108K69 -
 2:29:42
2:29:42
TheSaltyCracker
7 hours agoGhislaine Maxwell Talks ReEEeStream 7-25-25
98.7K205 -
 9:27
9:27
MattMorseTV
9 hours ago $3.84 earnedHe just lost EVERYTHING.
28K14 -
 5:52:21
5:52:21
a12cat34dog
8 hours agoSPOOKY ASS 2005 GAME :: F.E.A.R. :: FIRST-TIME FINISHING THIS CLASSIC {18+}
9.71K10