Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
Regulatory Affairs - eCTD Submission Gateways for FDA and EMA, and ICH-M8 (Pharmaceutical Sciences and Health Care products). Peivand Pirouzi, Ph.D.
6 months ago
25
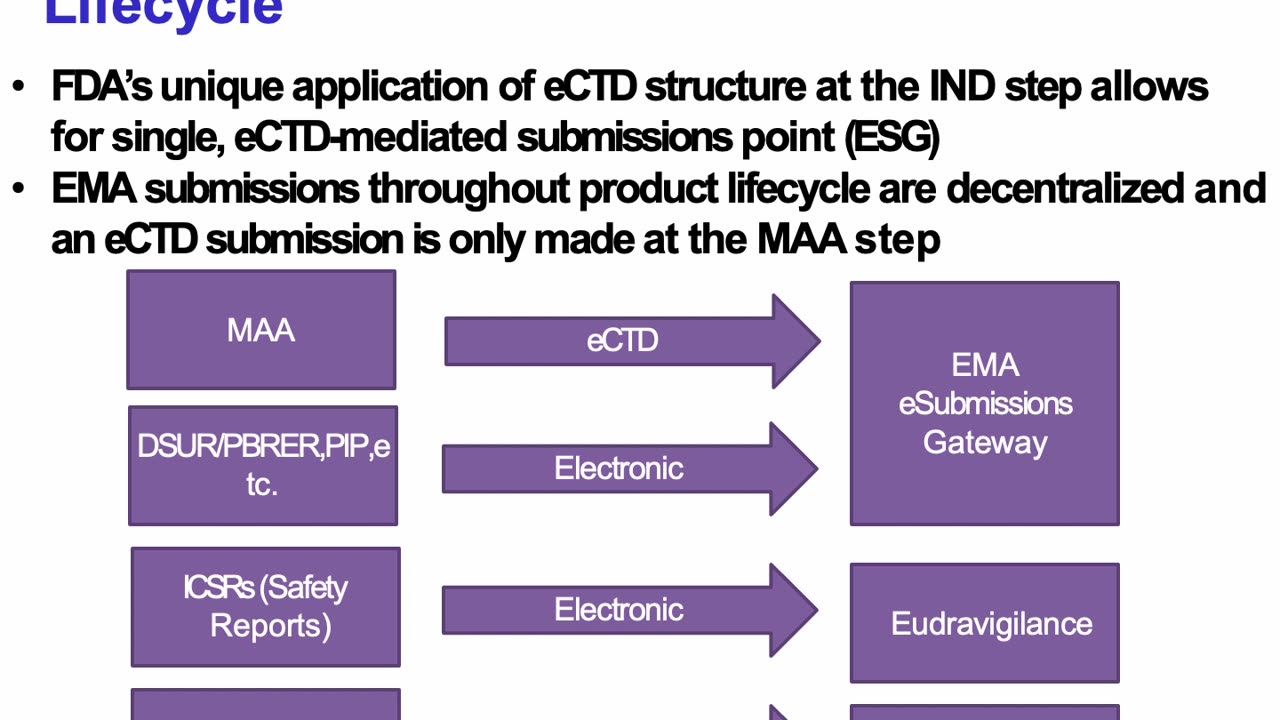
Discover the intricacies of submission gateways for regulatory compliance with the FDA and EMA in this comprehensive guide. In this video, we delve into the ICH-M8 guidelines, exploring how they streamline electronic submission processes for pharmaceutical and biotechnological products. Learn about:
Key features of the FDA’s Electronic Submission Gateway (ESG) and the EMA’s eSubmission Gateway/Web Client.
The role of ICH-M8 in harmonizing electronic standards across regulatory agencies.
Loading 1 comment...
-
 1:05:44
1:05:44
Omar Elattar
9 months agoThe Digital Real Estate Expert: You're Being Lied To About Making Money Online!
4.61K -
 10:53
10:53
Nikko Ortiz
2 days agoWORST Clips On The Internet
86K27 -
 3:42:38
3:42:38
FreshandFit
12 hours agoREAL R*pe Victim Exposes Shannon Sharpe Accuser As Liar!
70.1K74 -
 2:18:29
2:18:29
Badlands Media
14 hours agoDevolution Power Hour Ep. 376: Optics, Explosions & the War for the Narrative
144K43 -
 37:46
37:46
Stephen Gardner
12 hours ago🔥Trump NEVER expected THIS WIN as Schumer has EPIC MELTDOWN!
44K37 -
 2:02:41
2:02:41
Inverted World Live
8 hours agoNASA Engineer Says Trillions of Shape-Shifting, Cloaked Devices are Hidden on Earth| Ep. 83
39K11 -
 3:12:37
3:12:37
TimcastIRL
9 hours agoGOP Councilman DOUSED IN GAS, Set ON FIRE In Virginia, Suspect In Custody | Timcast IRL
250K91 -
 2:32:23
2:32:23
The Quartering
8 hours agoOn To The Big Bosses! Act 2 Of Expedition 33
63.1K6 -
 7:36:34
7:36:34
SpartakusLIVE
10 hours agoTiger Blood RESTOCKED and 30% off w/ code SPARTAKUS30
87.4K -
 24:58
24:58
Law&Crime
11 hours ago $3.55 earnedSecond Note Leaves Disturbing Clues in New York City Killings
40.5K12