Premium Only Content
Regulatory Affairs - Health Canada CTA in CTD format - Completing the clinical trial site information form. Peivand Pirouzi, Ph.D.
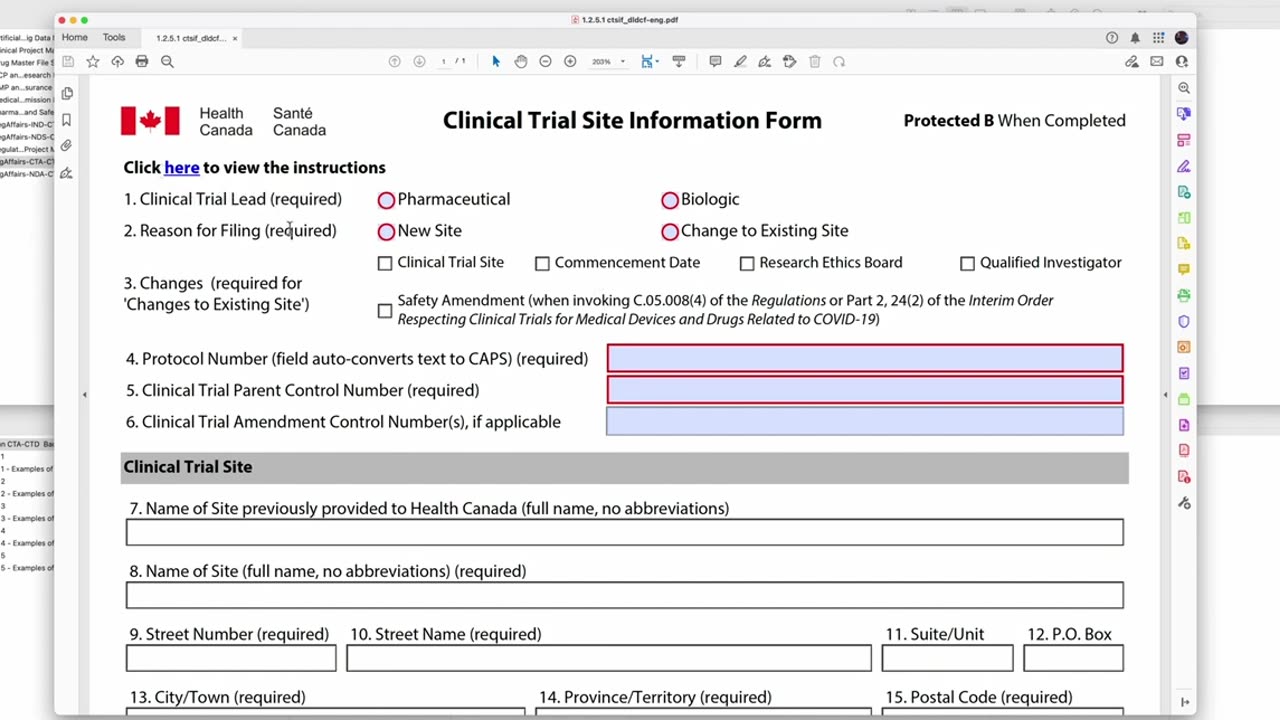
In this video, Professor Peivand Pirouzi provides a step-by-step guide on how to complete the Clinical Trial Site Information Form as part of the Clinical Trial Application (CTA) process to Health Canada, submitted in the Common Technical Document (CTD) format. This form is essential for providing detailed information about the clinical trial sites involved in the study, including the qualifications of the investigators and the capabilities of the facilities.
Key topics covered include:
An overview of the purpose and importance of the Clinical Trial Site Information Form in the CTA submission.
Detailed instructions on how to complete each section of the form, including site identification, investigator information, and site-specific requirements.
How to ensure the form complies with Health Canada regulations and expectations.
Tips for including accurate and complete site information to avoid delays in the review and approval process.
Common challenges when completing the form and how to overcome them effectively.
This video is invaluable for clinical trial managers, sponsors, and regulatory affairs professionals involved in submitting a CTA to Health Canada. By following Professor Pirouzi’s guidance, you will ensure the Clinical Trial Site Information Form is completed correctly, supporting the successful approval of your clinical trial application.
-
 LIVE
LIVE
StoneMountain64
1 hour agoHUNTING FOR THE FIRST WIN BACK ON WARZONE
372 watching -

Sean Unpaved
1 hour agoCFB Deep Dive: Matt Moscona's Expert Takes on the Gridiron
9.88K -
 27:39
27:39
Crypto.com
1 day ago2025 Live AMA with Kris Marszalek, Co-Founder & CEO of Crypto.com
84.8K4 -
 LIVE
LIVE
SternAmerican
22 hours agoElection Integrity Call – Wed, Aug 27 · 2 PM EST | Featuring Arizona
189 watching -
 1:00:05
1:00:05
Timcast
2 hours agoMASS SHOOTING At Catholic Church In Minneapolis, Children Reportedly Targeted
111K70 -
 1:34:01
1:34:01
Tucker Carlson
1 hour agoChristopher Caldwell: Is It Too Late to Save the English-Speaking World?
5.58K20 -
 2:14:40
2:14:40
Steven Crowder
4 hours agoBreaking: Minneapolis Catholic Church Shooting Live Coverage
314K295 -
 LIVE
LIVE
Major League Fishing
5 days agoLIVE! - Fishing Clash Team Series: Challenge Cup - Day 4
294 watching -
 25:03
25:03
Neil McCoy-Ward
1 hour agoFURY As JD Vance Unleashes HELL On The UK & EU… (What We Know So Far)
3.48K6 -
 LIVE
LIVE
IrishBreakdown
1 hour agoNotre Dame vs Miami Preview - Inside the Matchup
7 watching