Premium Only Content
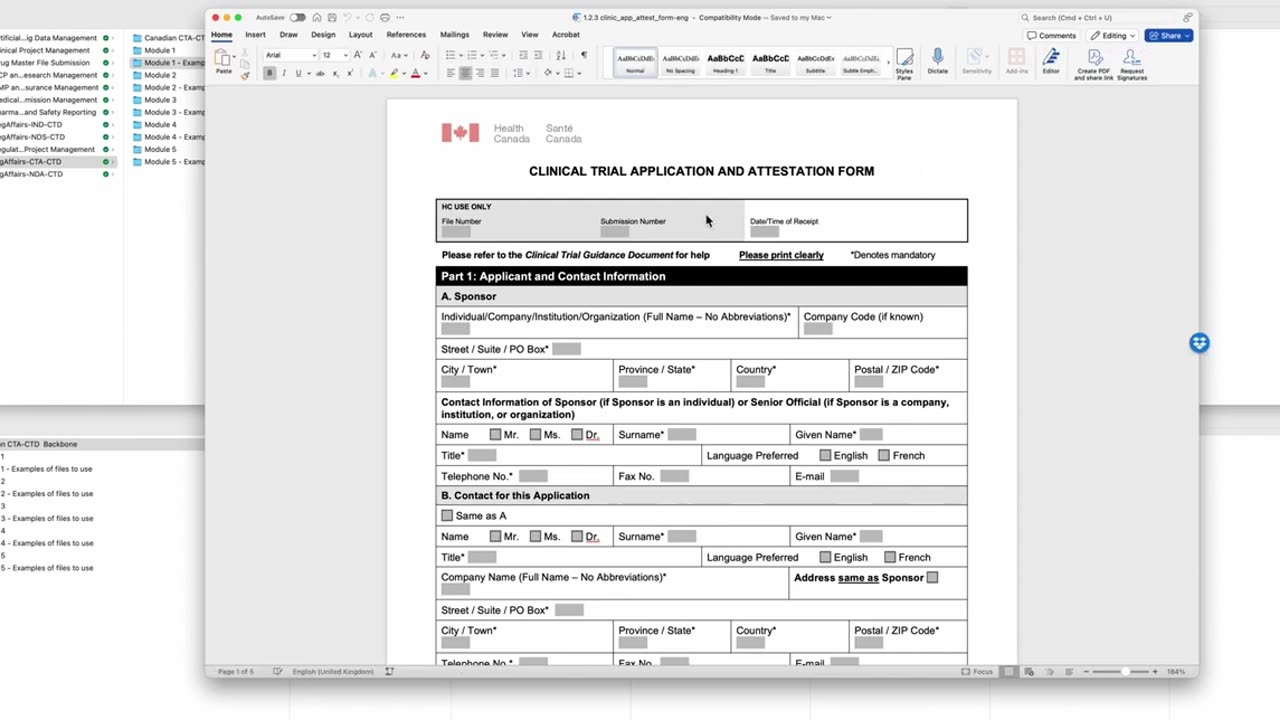
Regulatory Affairs - Health Canada CTA in CTD fromat - Completing the clinical trial attestation form. Peivand Pirouzi, Ph.D.
In this video, Professor Peivand Pirouzi provides an in-depth walk-through of how to complete the Clinical Trial Attestation Form as part of the Clinical Trial Application (CTA) submission to Health Canada in the Common Technical Document (CTD) format. This form is a critical component of the application, ensuring compliance with regulatory requirements for conducting clinical trials in Canada.
Key topics covered include:
An introduction to the Clinical Trial Attestation Form and its role in the CTA process.
Step-by-step guidance on filling out the form, including required information about the clinical trial, sponsor, and investigator.
Key sections of the form that need to be completed to confirm compliance with Health Canada regulations.
Common challenges in completing the form and how to avoid errors that could delay the application process.
Tips for submitting a complete and accurate CTA package to Health Canada, ensuring the timely approval of your clinical trial.
This video is an essential resource for regulatory affairs professionals, clinical trial managers, and anyone involved in the CTA process. With Professor Pirouzi’s guidance, you’ll learn how to properly complete the Clinical Trial Attestation Form, helping to streamline the submission process for clinical trials in Canada.
-
 LIVE
LIVE
JuicyJohns
1 hour ago🟢#1 REBIRTH PLAYER 10.2+ KD🟢$500 GIVEAWAY
120 watching -
 LIVE
LIVE
Outspoken with Dr. Naomi Wolf
15 hours ago"Jonathan Pollard: Are Allies of Israel being Targeted?"
75 watching -
 1:29:49
1:29:49
Game On!
17 hours ago $0.86 earned10,000 Followers Celebration Stream! Let's Talk Sports!
30.6K2 -
 15:38
15:38
SKAP ATTACK
19 hours ago $2.14 earnedA Legacy Tainted: Unpacking the LeBron Steroid Rumors
27.4K10 -
 2:04:52
2:04:52
The Confessionals
21 hours agoThe Great Spiritual Hijack — And Why It’s Ending
23.3K6 -
 1:49:30
1:49:30
Nick Freitas
15 hours agoHere's Why The Housing Market Is Permanently Broken
22.5K3 -
 17:03
17:03
Nicholas Bowling
19 hours ago $0.72 earned"Celebrity" Tells Street Preacher His Dad's in the ILLUMINATI
18.7K3 -
 2:01:09
2:01:09
BEK TV
22 hours agoTrent Loos in the Morning - 7/30/2025
18.2K -
 4:10
4:10
Blackstone Griddles
14 hours agoCajun Dogs with Bruce Mitchell
20.1K2 -
 29:38
29:38
Uncommon Sense In Current Times
15 hours ago $0.87 earnedIs Doubt a Sin? Wrestling with Faith & Belief (Part 1) | Dr. Randal Rauser
26.2K5