Premium Only Content
Regulatory Affairs - EMA - Marketing Authorization Applications for Pharmaceutical Products in EU. Peivand Pirouzi, Ph.D.
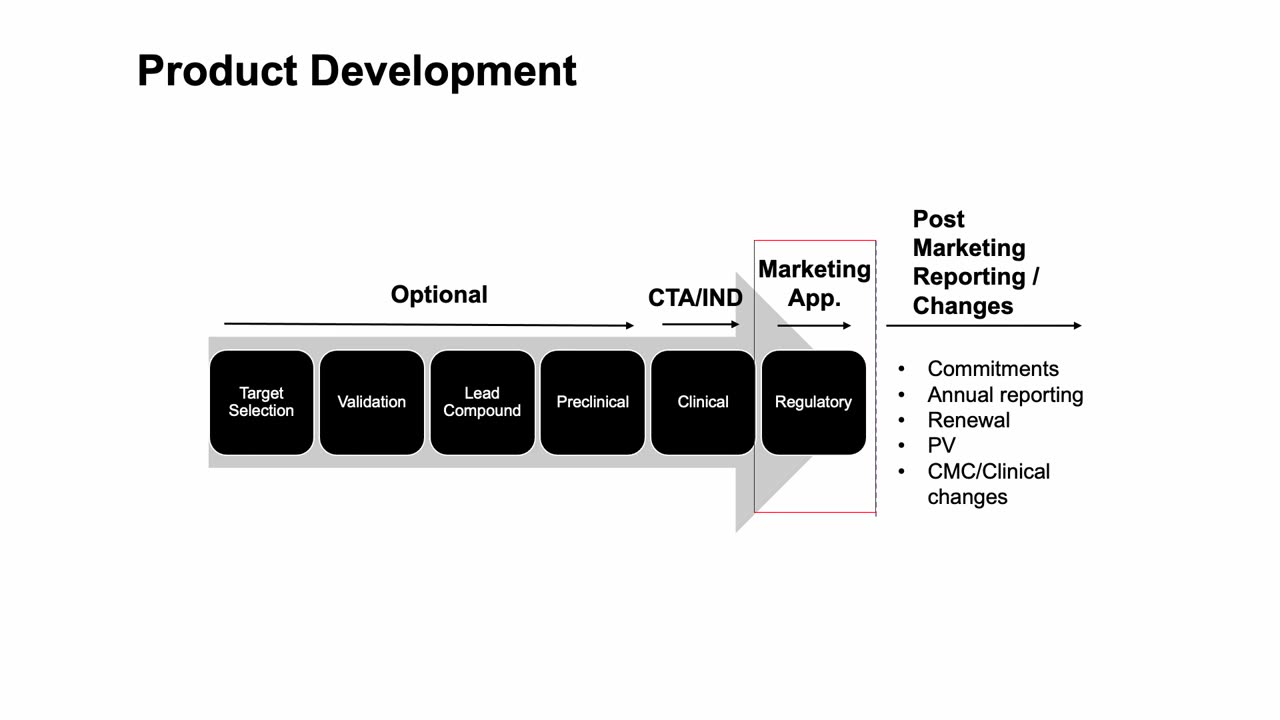
In this video, Professor Peivand Pirouzi provides an in-depth explanation of the Marketing Authorization Application (MAA) process for pharmaceutical products in the European Union (EU). The European Medicines Agency (EMA) plays a central role in regulating and approving medicinal products in the EU, and understanding the steps involved in submitting an MAA is crucial for pharmaceutical companies and regulatory affairs professionals.
Key topics covered include:
An overview of the EMA and its role in the marketing authorization process.
A detailed look at the Marketing Authorization Application (MAA) and the documents required for submission.
How to navigate the application process, from preparing the dossier to interacting with regulatory authorities.
Key requirements for demonstrating the quality, safety, and efficacy of pharmaceutical products in the EU market.
The importance of Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and other regulatory standards during the submission process.
Common challenges faced during MAA submissions and strategies for a successful outcome.
This video is an essential resource for pharmaceutical professionals, regulatory affairs specialists, and anyone interested in the EU drug approval process. Whether you’re preparing for your first MAA submission or seeking to improve your understanding of the process, this video offers valuable insights into the EMA’s procedures.
-
 2:29:34
2:29:34
Brandon Gentile
4 days agoHow To Retire 10 Years Early with Just 0.1 Bitcoin
1.11K1 -
 3:19:34
3:19:34
Laura Loomer
5 hours agoEP135: Champagne Communism: Zohran Mamdani's Ugandan Compound EXPOSED
28.4K7 -
 28:39
28:39
The Why Files
3 days agoCryptids Vol. 4 | Bunyips, Yowie and Australian Nightmare Fuel
45.6K38 -
 1:07:06
1:07:06
Mike Rowe
18 days agoThe Fight For America's Heartland | Salena Zito #442 | The Way I Heard It
30.3K47 -
 2:43:30
2:43:30
TimcastIRL
6 hours agoSouth Park Goes FULL CHARLIE KIRK, Latest Episode ROASTS Trump Again | Timcast IRL
210K79 -
 LIVE
LIVE
SpartakusLIVE
6 hours agoThe Return of the KING of Content
425 watching -
 10:05
10:05
MattMorseTV
9 hours ago $6.53 earnedHe actually did it...
55.1K23 -
 1:32:39
1:32:39
Anthony Rogers
1 day agoEpisode 376 - Todd Schowalter
25.7K -
 3:42:07
3:42:07
megimu32
5 hours agoOTS: Movie Tie-In Games + Remakes: Let’s Play Memory Lane
41.1K5 -
 1:15:06
1:15:06
Adam Does Movies
13 hours ago $1.02 earnedTalking Movies + Ask Me Anything - LIVE
28.6K1