Premium Only Content
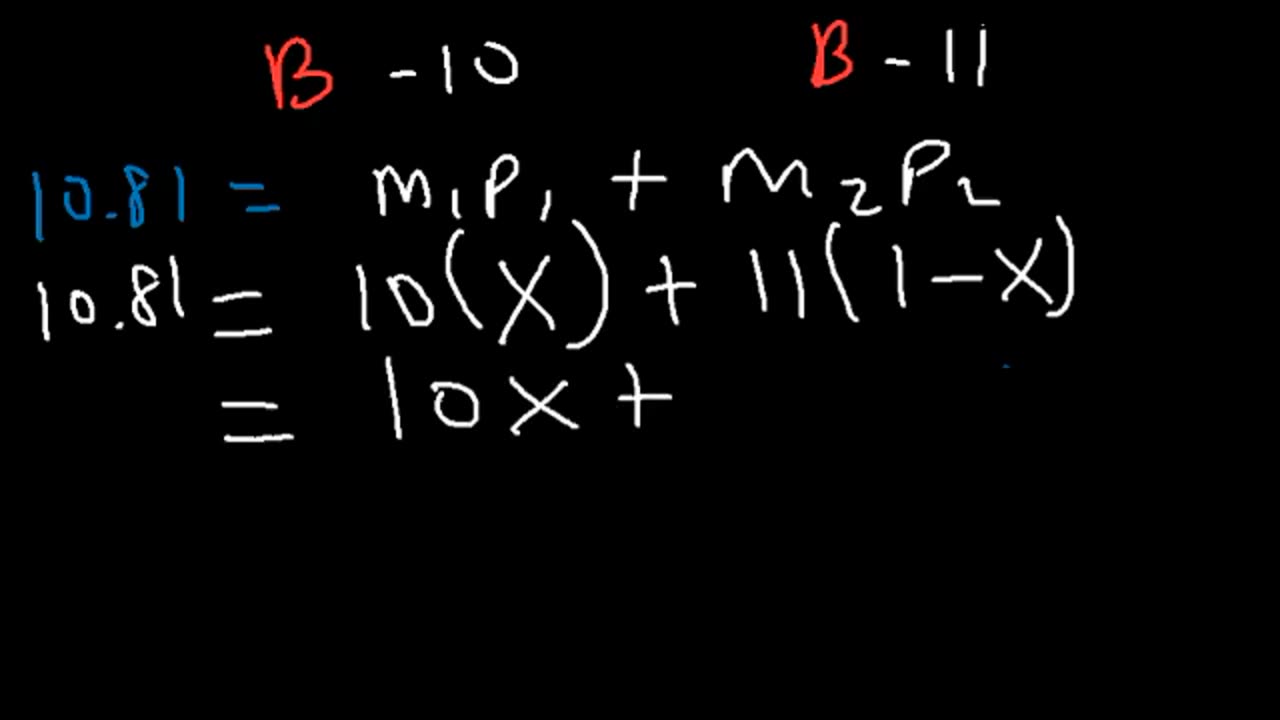
Average Atomic Mass Practice Problems
This chemistry video tutorial shows you how to calculate the average atomic mass of 2 or 3 isotopes. It provides the equation / formula for you to do so. In addition, it also shows you how to calculate the relative percent abundance or percent natural abundance of isotope using the weighted average atomic mass. This video provides plenty of examples and practice problems for you to work on.
Chemistry - Basic Introduction:
• Chemistry
Significant Figures Review:
• Significant Figures - A Fast Review!
Unit Conversion Problems:
• Converting Units With Conversion Fact...
Pure Substances & Mixtures:
• Pure Substances and Mixtures, Element...
Physical and Chemical Changes:
• Physical and Chemical Changes
_________________________________
Atoms - Basic Introduction:
• Atoms - Basic Introduction
Cations and Anions Explained:
• Cations and Anions Explained
Diatomic Elements & Molecules:
• Diatomic Elements & Molecules
Elements, Atoms, & Molecules:
• Elements, Atoms, Molecules, Ions, Ion...
Protons, Neutrons, & Electrons:
• How To Calculate The Number of Proton...
__________________________________
Average Atomic Mass:
• How To Calculate The Average Atomic Mass
What Are Isotopes?
• What are Isotopes?
Percent Abundance of Isotopes:
• How To Find The Percent Abundance of ...
Ionic and Covalent Bonding:
• Ionic and Covalent Bonding - Chemistry
Naming Molecular Compounds:
• How To Name Covalent Molecular Compou...
Writing Formulas - Ionic Compounds:
• Writing Chemical Formulas For Ionic C...
__________________________________
Final Exams and Video Playlists:
https://www.video-tutor.net/
Full-Length Videos and Worksheets:
-
 1:04:05
1:04:05
TheOrganicChemistryTutor
6 months agoChemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry
661 -
 LIVE
LIVE
Dr Disrespect
6 hours ago🔴LIVE - DR DISRESPECT - 10 WINS CHALLENGE - BIG ANNOUNCEMENT AT 12PM PT
2,022 watching -
 LIVE
LIVE
Barry Cunningham
1 hour agoPRESIDENT TRUMP MAKES SPEECH ABOUT AI
1,637 watching -
 LIVE
LIVE
The Jimmy Dore Show
20 minutes agoJon Stewart LIVID About Colbert Show Cancelation! Trump ADMITS His Name Is in the Epstein Files!
2,380 watching -
 13:18
13:18
The Rad Factory
8 hours ago $0.02 earnedIs This E Scooter Worth $900?
73 -
 54:38
54:38
The Dr. Ardis Show
6 hours ago $1.52 earnedThe Dr. Ardis Show | 5 Ways Alcohol Destroys Your Health | Episode 07.23.2025
1.83K3 -
 LIVE
LIVE
RalliedLIVE
5 hours ago $1.36 earned10 WINS WITH THE SHOTTY BOYS
117 watching -
 1:14:33
1:14:33
Dr. Drew
5 hours agoBenny Johnson & Mama June: America & Families In Crisis w/ Pumpkin & Jessica (The Double Feature Nobody Expected) – Ask Dr. Drew
14.2K -
 4:00:56
4:00:56
Viss
4 hours ago🔴LIVE - How to Consistently Win in PUBG!
9011 -
 DVR
DVR
StoneMountain64
6 hours agoHIDE AND SEEK. IM GOING TO FIND YOU.
530