Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
Collision Model, Activation Energy, Example - Chemistry
9 months ago
36
Health & Science
Education
activation energy
collision theory
chemistry
collision theory chemistry
activation energy chemistry
collision theory and activation energy
collision theory of chemical kinetics
collision model
collision theory and rates of reaction
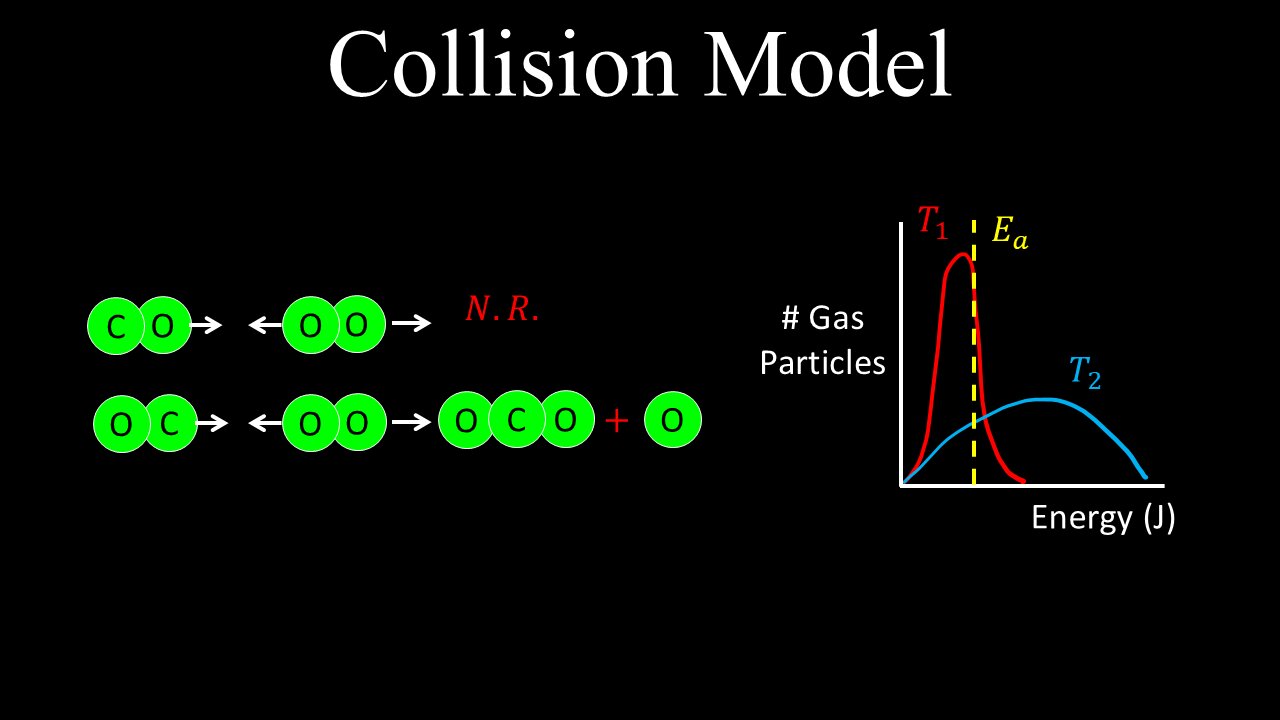
This video explains the collision model or theory of reaction rates, including what the collision particle theory is, and the Maxwell-Boltzmann distribution curve. The Arrhenius equation is deferred to another tutorial. The collision model determines if a reaction is likely to occur. The Maxwell-Boltzmann distribution is used to visualise the number of particles at different temperatures that are likely to overcome the activation energy required to initiate the reaction.
Loading 1 comment...
-
 LIVE
LIVE
The Bubba Army
22 hours agoGhislaine Maxwell to Testify! - Bubba the Love Sponge® Show | 7/24/25
2,390 watching -
 16:50
16:50
Chris From The 740
1 hour agoI Didn’t Expect to Like This Fan So Much – Ogery F11 Review!
2.48K -
 8:11
8:11
Millionaire Mentor
16 hours agoJohn James SHUTS DOWN AOC With One BRUTAL Sentence
13.2K17 -
 31:34
31:34
Friday Beers
15 hours ago $2.61 earnedOur Horrifying Night Drunk Ghost Hunting the Manson Murders
42.4K7 -
 4:09
4:09
Blackstone Griddles
14 hours agoEndless Summer Smashburgers on the Blackstone Griddle
16.9K5 -
 LIVE
LIVE
BEK TV
23 hours agoTrent Loos in the Morning 7/24/2025
189 watching -
 7:02
7:02
China Uncensored
16 hours agoWell, I Guess Now We Know...
16.5K22 -
 46:10
46:10
Members Club
19 hours ago $0.94 earnedThe WNBA Has Demands, TSA Loosens Up, and NYC Has a Whale Whisperer - MC04
14K4 -
 1:35:15
1:35:15
Man in America
15 hours ago🚨 ALERT: Hospitals in the U.S. Are KILLING Patients… for Their Organs!
88.9K39 -
 8:00
8:00
DropItLikeItsScott
16 hours ago $1.06 earnedIs This The BEST SIG P365? SIG P365 FUSE
11.8K