Premium Only Content
Illegal "Pseudo-Laws" Allowed the U.S. Gov’t to Deploy C-19-Injection Bioweapons on Americans
and Given Legal Immunity to Those Who Administer The Kill Shots (Tweet 1/9)
💉THE POISON NEEDLE AND THE LEGAL SHIELD🛡️
Although many prominent voices in the “health freedom movement” espouse the idea that the C19 injections are pharmaceutical products, they are not. This is unequivocal. Therefore, they are beholden to no FDA regulations, and cannot be regulated by the FDA.
In a pair of videos released earlier this year, retired pharmaceutical industry R&D executive Sasha Latypova (@sasha_latypova
) describes, in detail—with all “the receipts”—how the C19 injections are, in fact, in a class of their own, so to speak; one NOT REGULATED BY THE FDA.
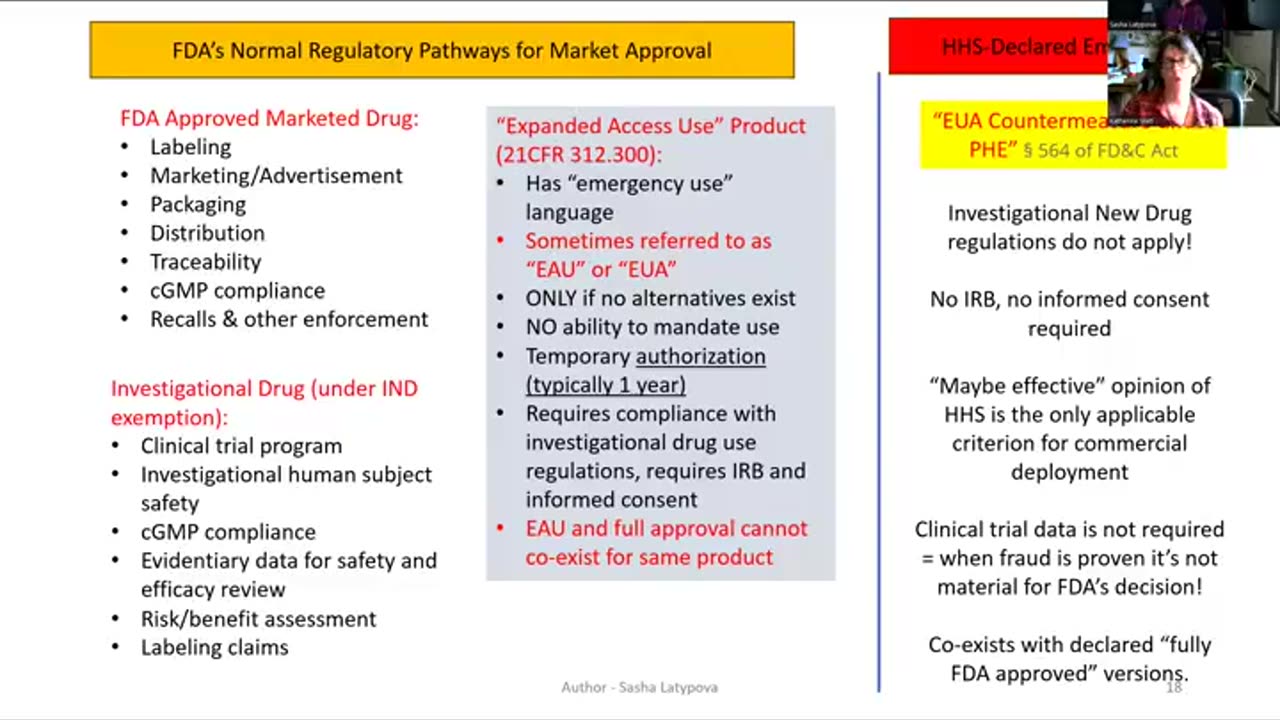
In this first clip from a conversation between Latypova and writer and paralegal Katherine Watt, Latypova explains how there are three regulatory pathways for pharmaceutical drugs, which are “normal regulatory pathways, where we don’t have [an] emergency announcement.” Latypova notes that these pathways include investigational use of the product; FDA-approved drugs; investigational drugs (if you want to introduce a new drug, then you need to clinical trial it across state lines); and drugs made available under “expanded access use.”
Expanded access use, Latypova notes, is “a more recent evolution” of regulatory law, which was put into place in 1997. Since then, expanded access use has allowed for the use of experimental drugs in “desperate situations.”
The fourth regulatory pathway is the one used for the C19 injections: “EUA countermeasures under [a] public health emergency.” Latypova notes that for an “EUA under public health emergency, none of these normal [FDA regulations] apply [in an enforceable manner].” This is why Latypova and Watt refer to the supposed FDA regulation and approval of the C19 injections as “performance art.”
The injections are EUA countermeasures deployed under a public health emergency. Per the law, they cannot be regulated as pharmaceutical drugs.
Specifically, Latypova notes that there is “no requirement for [an] IRB [an institutional review board] or informed consent…” She adds, “investigational new drug regulations don’t apply, [and] clinical trial data is not required.”
Latypova notes that this is why when clinical trial investigator Brook Jackson (@IamBrookJackson
) observed fraud at Pfizer’s C19-injection clinical trial sites in Texas, reported it, and started litigating, the judge dismissed her case—because any fraud that existed was “immaterial.”
Sensereceptor : https://x.com/SenseReceptor/status/1732229248868303162
also see: 'Gates/DARPA's search for extinction gene'
https://genedrivefiles.synbiowatch.org/
-
 LIVE
LIVE
TimcastIRL
1 hour agoNew DOCS PROVE Obama Hillary CONSPIRACY To SABOTAGE Trump Admin | Timcast IRL
20,425 watching -
 21:53
21:53
Glenn Greenwald
3 hours agoMichael Tracey on the Street: What Do People Think of the Epstein Case?
70.2K27 -
 LIVE
LIVE
DamnDanieI
57 minutes agoKill First, Loot Later – OTG Live
502 watching -
 LIVE
LIVE
SpartakusLIVE
1 hour agoDuos w/ @GloryJean || #1 Masculine Muscle MASS sears YOUR retinas with MIND BENDING content
130 watching -
 56:41
56:41
Donald Trump Jr.
5 hours agoLies, Leaks, and Lawfare: Censorship Corruption Exposed | TRIGGERED Ep.263
98.9K99 -
 1:26:13
1:26:13
Mally_Mouse
3 hours agoLet's Hang!! -- P.O. Box & Chill!
1.54K -
 1:02:37
1:02:37
BonginoReport
4 hours agoKamala Teases Book About Dumpster Fire Campaign - Nightly Scroll w/ Hayley Caronia (Ep.102)
47.3K33 -
 35:05
35:05
Stephen Gardner
4 hours ago🔥Obama will be FORCED to Testify in Trump trial?
11.1K23 -

Tundra Tactical
1 hour agoTundra Tactical Political Memes Review!
4.46K -
 LIVE
LIVE
GloryJean
1 hour agoDuos w/ Spartakus 🔥 LOCK IN for Games w/ PASSION
18 watching