Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
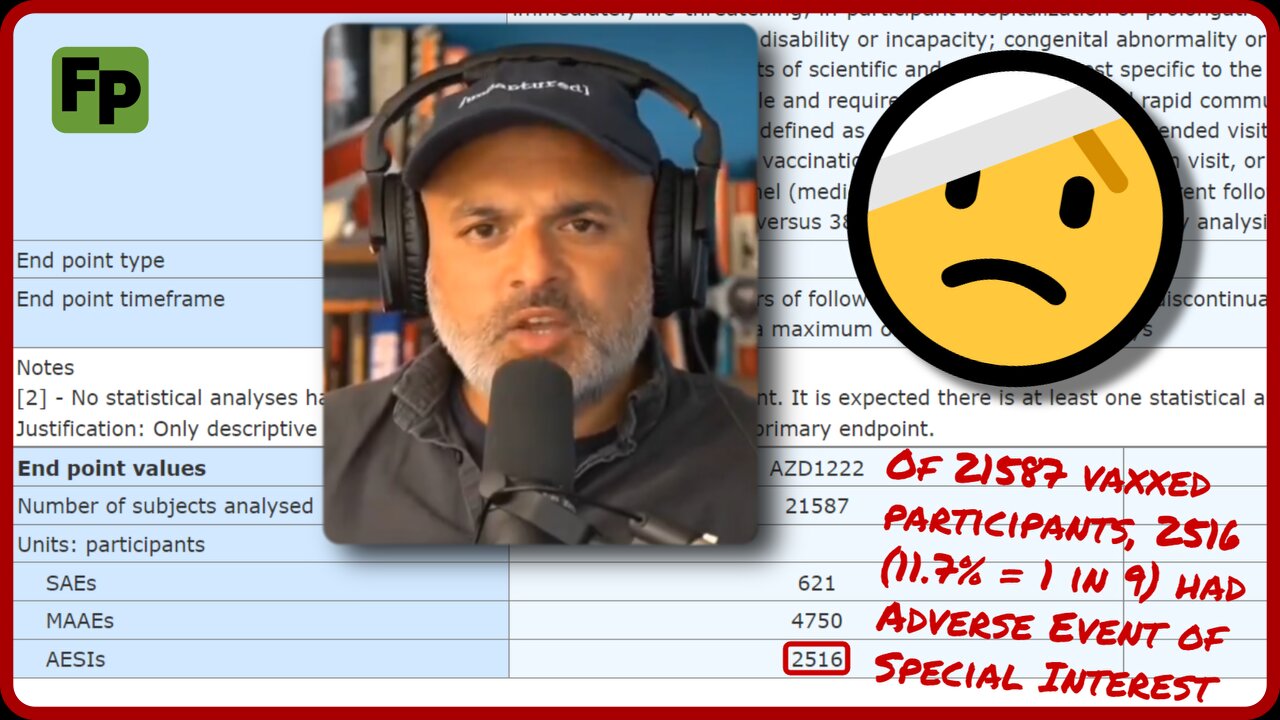
1 in 9 AstraZeneca trial participants had Adverse Event of Special Interest | Dr. Ahmad Malik
Repost
6 months ago
588
REFERENCES
Original interview (I used about two thirds of the interview in this video):
https://x.com/james_freeman__/status/1791545328832827827
Definition of AESI is in tabel II-9 (pp. 37-8) of the EMA document (linked below), and in this tweet:
https://x.com/eh_den/status/1792111953168548286
Article by Ehden Biber:
https://ehden.substack.com/p/the-long-term-safety-study
European Medicines Agency (EMA) risk management plan:
https://www.ema.europa.eu/en/documents/rmp-summary/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-risk-management-plan_en.pdf
Clinical Trial register:
https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-005226-28/results
Tweet by Andrew Bridgen:
https://x.com/ABridgen/status/1792071396425691322
Loading 2 comments...
-
 LIVE
LIVE
TheAlecLaceShow
3 hours agoGuests: Senator Tommy Tuberville & Asm. Brian Bergen | NJ Drones | Trump Time | The Alec Lace Show
79 watching -
 59:24
59:24
The Dan Bongino Show
4 hours agoWhat Is The Government Hiding From Us? (Ep. 2387) - 12/12/2024
487K1.49K -
 1:39:33
1:39:33
Benny Johnson
2 hours ago🚨Libs FREAK As Trump Puts Kari Lake in Charge of American Media, Trump WINS Time's 'Person of Year'
38.1K48 -
 1:06:08
1:06:08
The Rubin Report
2 hours agoRogan Is Outraged When He Finds Out Dems' Unexpected Pardon Plans
40K24 -
 1:59:13
1:59:13
Steven Crowder
4 hours ago🔴 Trump is Time's Person of the Year & The Deep State Fails at Smearing Pete Hegseth
292K168 -
 LIVE
LIVE
The Shannon Joy Show
5 hours ago🔥🔥LIVE Exclusive With James Lindsay! Beware The WOKE RIGHT - A Marxist Counter Movement To Woke Left🔥
405 watching -
 DVR
DVR
The Nima Yamini Show
2 hours agoWill Germany Take Another 1 Million Syrians in 2025? Join the podcast with Miró
8.33K1 -
 34:38
34:38
Rethinking the Dollar
2 hours agoThe Melt Up 2025 Will Be Jaw Dropping | Morning Check-In
8.09K2 -
 1:14:23
1:14:23
Graham Allen
5 hours agoIran Drones Enter The US!! Is This WWIII?! + Trump Named MAN OF THE YEAR!
97K103 -
 LIVE
LIVE
hambinooo
4 hours agoBRAND NEW AWESOME FPS GAME
297 watching