Premium Only Content
Le Chatlier's Pranciple | Effect of concentration | Effect of Pressure| Effect of Temperature
Description:
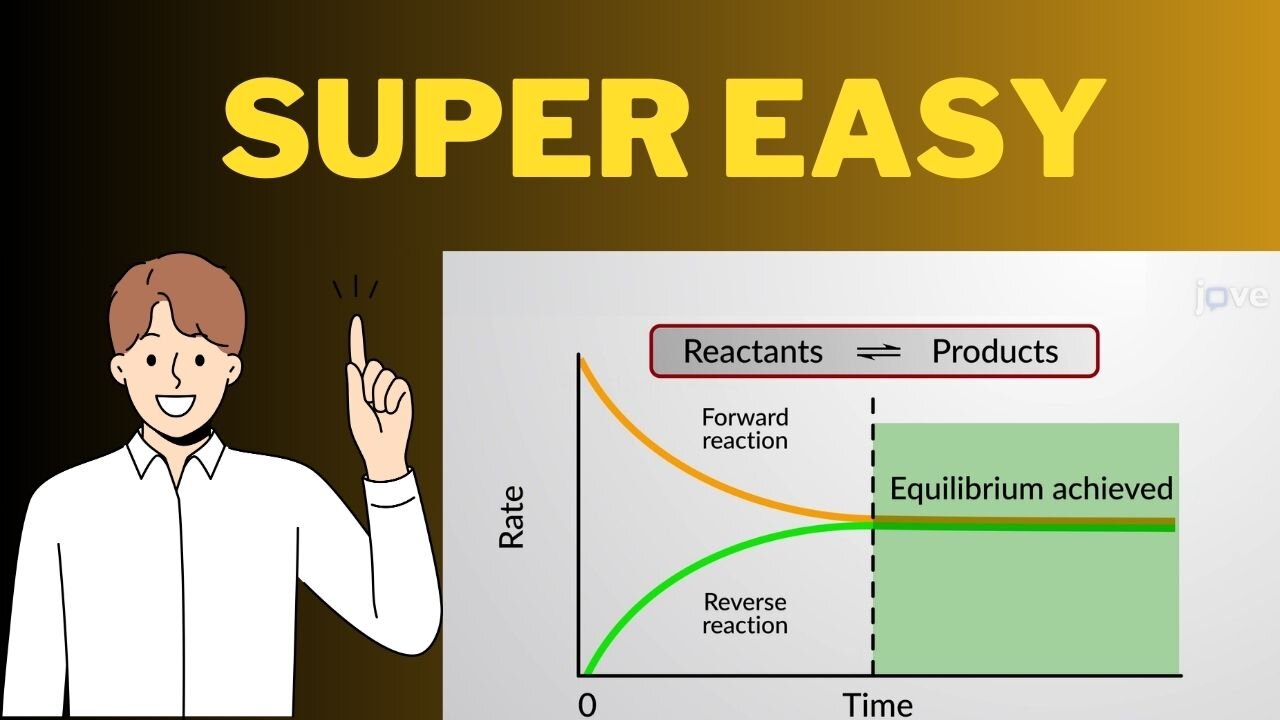
Welcome to "Le Chatelier's Principle: Exploring the Effects of Concentration, Pressure, and Temperature." In this video, we delve into the fundamental concepts of chemical equilibrium and investigate how changes in concentration, pressure, and temperature influence the equilibrium position of a reaction, as described by Le Chatelier's Principle.
Firstly, we'll explore the effect of concentration on a system at equilibrium. By adding or removing reactants or products, we can disrupt the equilibrium and observe the system's response as it adjusts to restore equilibrium. Through visual demonstrations and explanations, we'll illustrate how changes in concentration shift the equilibrium position, ultimately reaching a new balance.
Next, we'll examine the impact of pressure changes on equilibrium. For gaseous reactions, alterations in pressure can significantly affect the equilibrium state. By altering the volume of the container or adjusting the number of moles of gas involved, we can observe how the equilibrium shifts to counteract changes in pressure, following Le Chatelier's Principle.
Finally, we'll investigate the influence of temperature on chemical equilibrium. Temperature changes can either favor the forward or reverse reaction, depending on whether the reaction is exothermic or endothermic. We'll demonstrate how altering the temperature affects the equilibrium constant and explore the factors influencing this dynamic equilibrium.
Throughout the video, we'll provide detailed explanations, real-life examples, and visual representations to help you grasp the intricate interplay between concentration, pressure, and temperature on chemical equilibrium, as governed by Le Chatelier's Principle.
Join us on this educational journey as we unravel the mysteries of equilibrium and deepen our understanding of Le Chatelier's Principle. Don't forget to like, share, and subscribe for more insightful content on chemistry and science!
-
 1:06:09
1:06:09
Man in America
16 hours agoExposing HAARP's Diabolical Mind Control Tech w/ Leigh Dundas
63.9K55 -
 1:47:16
1:47:16
Tundra Tactical
11 hours ago $105.36 earnedGlock Interview From Beyond The Grave//Whats the Future of Home Training??
52.2K8 -
 2:16:35
2:16:35
BlackDiamondGunsandGear
10 hours agoEBT Apocalypse? / Snap Down SHTF / After Hours Armory
21.9K11 -
 14:05
14:05
Sideserf Cake Studio
21 hours ago $15.62 earnedHYPERREALISTIC HAND CAKE GLOW-UP (Old vs. New) 💅
55.7K11 -
 28:37
28:37
marcushouse
23 hours ago $8.93 earnedSpaceX Just Dropped the Biggest Starship Lander Update in Years! 🤯
29.5K10 -
 14:54
14:54
The Kevin Trudeau Show Limitless
3 days agoThe Hidden Force Running Your Life
109K25 -
 2:16:35
2:16:35
DLDAfterDark
10 hours ago $10.00 earnedIs The "SnapPocalypse" A Real Concern? Are You Prepared For SHTF? What Are Some Considerations?
28.1K11 -
 19:58
19:58
TampaAerialMedia
21 hours ago $9.45 earnedKEY LARGO - Florida Keys Part 1 - Snorkeling, Restaurants,
44.1K21 -
 1:23
1:23
Memology 101
2 days ago $8.80 earnedFar-left ghoul wants conservatives DEAD, warns Dems to get on board or THEY ARE NEXT
35K71 -
 3:27:27
3:27:27
SavageJayGatsby
11 hours ago🔥🌶️ Spicy Saturday – BITE Edition! 🌶️🔥
61.2K7