Intermolecular Forces - Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions
This chemistry video tutorial focuses on intermolecular forces such hydrogen bonding, ion-ion interactions, dipole dipole, ion dipole, london dispersion forces and van deer waal forces. It contains plenty of examples and practice problems to help you understand the most important concepts related to this material.
Chemistry Basic Introduction and Final Exam Review:
https://www.video-tutor.net/chemistry...
My Twitter Page:
https://twitter.com/OrgoChemTutor21
Here is a list of topics:
1. Ion - Ion dipole interactions of KF and CaO
2. Electrostatic Force and Lattice Energy- The effect of charge and ionic radii or size
3. How To Determine Which Ionic Compound has a Higher Melting Point - NaF vs KCl
4. Ion-Dipole Interactions - NaCl and H2O
5. Definition of a Dipole - Polar Molecules & Charge Separation
6. Dipole-Dipole Interactions of Polar Molecules - Partial Charge Electrostatic Attractions of CO
7. Hydrogen Bonding between Hydrogen, Nitrogen, Oxygen, and Fluorine
8. Intermolecular Forces vs Intramolecular Forces
9. Hydrogen Bonding vs Polar & Nonpolar Covalent Bonds
10. London Dispersion Forces & Van Der Waals Forces
11. Permanent Dipoles and Temporary Induced Dipoles - Distribution of electrons in electron cloud
12. Difference Between Atoms and Ions - Cations vs Anions - Number of Electrons and Protons
13. The relationship between Polarizability and Dispersion Forces
14. How To Determine the Strongest Intermolecular Forces In Compounds Such as MgO, KCl, H2O, CH4, CO2, SO2, HF, CH3OH, LiCl, CH2O, CO, and I2
15. The relationship between Boiling Point and Vapor Pressure
16. Straight Chained vs Branched Alkanes - Boiling Point and Intermolecular Forces - Surface Area
17. Ranking Boiling Point In Order of Increasing Strength for I2, Br2, F2, and Cl2
18. Polar and Nonpolar Organic Compounds - Polarity and Water Solubility
19. Ranking Boiling In Decreasing Order For HF, HCl, HBr, and HI
20. The effect of Molar Mass and Number of electrons on the Overall Intermolecular Force / LDF
-
 32:50
32:50
Math Easy Solutions
1 month ago $0.24 earnedChemical Basis of Biology: Atoms, Molecules, and Electromagnetism
2242 -
 19:31
19:31
Math Easy Solutions
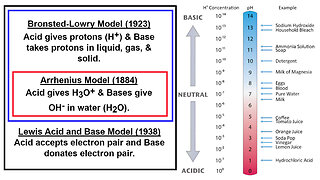
1 month ago $0.07 earnedAcids and Bases: Brønsted–Lowry, Arrhenius, and Lewis Models
1821 -
 20:21
20:21
Math Easy Solutions
1 month ago $0.18 earnedImportant Chemistry Terms
1481 -
 16:08
16:08
FanaticVoyage
3 months agoExperiments with the Bubble Model of Metal Structure 1952 - Sir Lawrence Bragg, W.M Lomer, J.F. Nye
66 -
 1:32
1:32
DrOfEng
1 month agoCoulomb Force, Bohr Model, Atoms - AP Chemistry
2 -
 2:41
2:41
Calihault
7 months agoChemical Bonds: A Journey from Strongest to Weakest
36 -
![[2017] Nanoparticle Corona Lecture (Permanent sensors)](https://hugh.cdn.rumble.cloud/s/s8/1/u/L/T/r/uLTrq.0kob-small-2017-Nanoparticle-Corona-Le.jpg) 1:06:26
1:06:26
Censored Important Videos
3 months ago[2017] Nanoparticle Corona Lecture (Permanent sensors)
924 -
 2:16
2:16
DrOfEng
3 months agoMoles, Connecting Units - AP Chemistry
5 -
![Nanoparticle Corona for Sensors [2017]](https://hugh.cdn.rumble.cloud/s/s8/1/F/s/X/r/FsXrq.0kob-small-Nanoparticle-Corona-for-Sen.jpg) 16:28
16:28
Censored Important Videos
3 months agoNanoparticle Corona for Sensors [2017]
1.33K8 -
 0:34
0:34
ShortScience
7 months agoUnlocking the Secrets of Molecules: Diastereoisomers Demystified
57