Premium Only Content
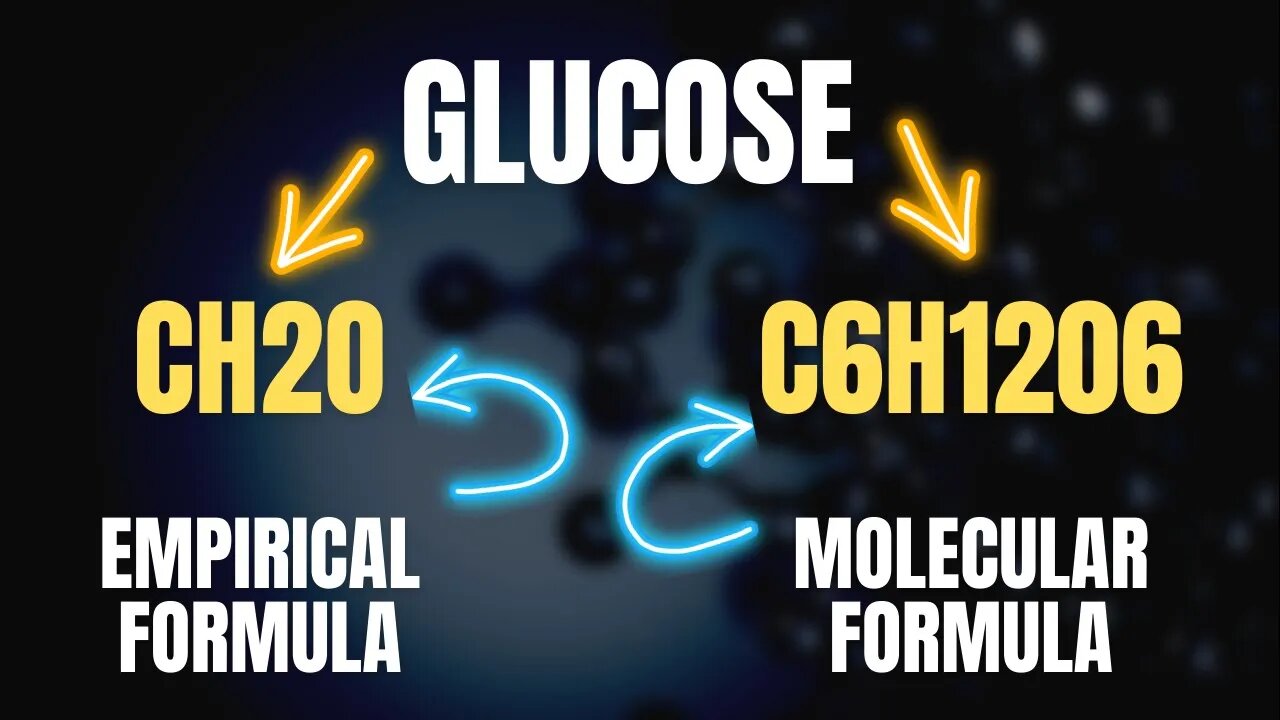
Empirical and Molecular Formula Explained
#chemistry #empiricalformula #molecularformula
In this video, we will be diving into the world of Empirical and Molecular formulas. These concepts are essential for students in Class 9 and 11 and are often covered in chemistry curriculums. We will begin by explaining the concept of an empirical formula, which is the simplest whole number ratio of atoms in a compound. Through clear explanations and examples, we will then demonstrate how to calculate the empirical formula of a compound.
Next, we will cover the concept of a molecular formula, which represents the actual number of atoms in a molecule. We will also explain the difference between empirical and molecular formulas, and how to convert from one to the other. We will also provide several examples to help you understand the concepts better.
So whether you're looking to improve your understanding of these important concepts or simply want to get ahead in class, this video is perfect for you. With clear explanations, helpful examples, and step-by-step instructions, you'll be able to master the concepts of Empirical and Molecular formulas in no time.
~ Study material ~
https://drive.google.com/file/d/18WTg5e2XmVIgVtPbdPChgTCyXjtch1JC/view?usp=drivesdk
-

X22 Report
3 hours agoMr & Mrs X - Tylenol Is The Start, All Roads Lead To The Vaccines, Chatter Amongst Big Pharma - Ep 9
43K8 -
 35:24
35:24
The Rubin Report
20 hours agoThe Simple Rules to Fight Crime Blue Cities Choose to Ignore | Jay Collins
22.5K14 -
 LIVE
LIVE
FyrBorne
10 hours ago🔴Warzone M&K Sniping: Modern Warfare Weapons That Still Cook in 2025
3,799 watching -
 17:40
17:40
Actual Justice Warrior
1 day agoBlack Mob ATTACKS Conservatives On Campus
79.7K171 -
 2:04:41
2:04:41
MG Show
23 hours agoJames 'Dirty Cop' Comey Indicted; A Plan to Starve the American People
62.8K21 -
 9:11
9:11
MattMorseTV
19 hours ago $23.30 earnedVance just DROPPED the HAMMER.
147K86 -
 10:16
10:16
GritsGG
19 hours agoBEST Controller Settings for Warzone! Rank 1 Player's Settings!
41.8K4 -
 2:13:30
2:13:30
Side Scrollers Podcast
1 day agoUK Introduces MANDATORY Digital ID + Dallas ICE Shooting BLAMED on Gaming + More | Side Scrollers
163K24 -
 10:34
10:34
The Pascal Show
19 hours ago $8.32 earnedFOOTAGE REVEALED! Images Of Celeste Rivas Exposed Before Her Disappearance From Home Running To D4vd
53.3K3 -
 LIVE
LIVE
Lofi Girl
2 years agoSynthwave Radio 🌌 - beats to chill/game to
288 watching