Premium Only Content
How to find percentage yield| theoretical yield| Actual yield|2020|AIM Pharma
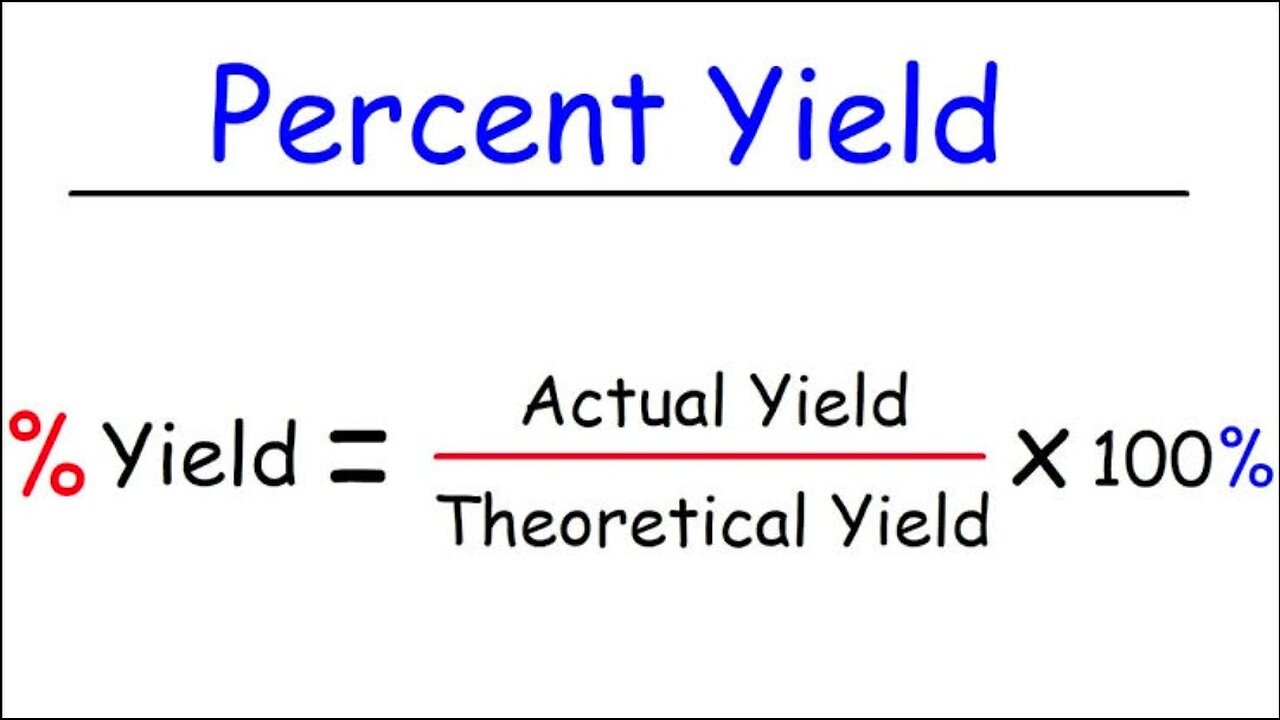
Percent Yield is defined as the actual yield divided by the theoretical yield times 100. Percent Yield=(Actual YieldTheoretical Yield)×100% There are many reasons why the actual yield of a chemical reaction may be less than the theoretical yield, and these will be taken up during later Chapters of the course.
Percent Yield
Percent Yield is defined as the actual yield divided by the theoretical yield times 100.
Percent Yield=(Actual YieldTheoretical Yield)×100%(4.3.1)
There are many reasons why the actual yield of a chemical reaction may be less than the theoretical yield, and these will be taken up during later Chapters of the course. Here are some reasons, most of which deal with topics that will be covered in the second semester of this course.
Equilibria between products and reactants, where the limiting reagent is not completely consumed (Chapter 15)
Ca3PO4(s)⇋3Ca+2+2PO−34(4.3.2)
Kinetics, simply speaking, the reaction may be very slow and not over (Chapter 14).
Formation of Intermediates. A chemical equation does not represent the mechanism but the overall balance of mass. The mechanism tells how the reaction actually proceeds (section 14.6), and there are often intermediate chemical species that are not completely consumed. This is exemplified in the following reaction.
H2(g)+2ICl(g)→I2(g)+2HClg(4.3.3)
It is highly unlikely that this reaction takes place in one step where three molecules simultaneously collide. A more plausible way for the reaction to occur is in two steps as below, where HI is an intermediate.
H2+IClHI+ICl→HCl+HI→HCl+I2(4.3.4)(4.3.5)
In the first step HCl and the intermediate HI are formed from the bimolecular collision of H2 and ICl. In the second step, the HI (intermediate) collides with another ICl to form HCl and I2. If you add the two steps the intermediate cancels out and you get the balanced equation.
H2(g)+2ICl(g)→I2(g)+2HClg(4.3.6)
4. Competing or Parallel Reactions
You may have a secondary reaction competing with the reaction described, like the formation of hydroiodic acid and chlorine gas.
H2+IClHCl+ICl→HI+HCl→HI+Cl2(4.3.7)(4.3.8)
So any HI or Cl2 formed would reduce the yield of the desired I2 and HCl. A parallel or competing reaction could also involve a completely different reactant. For example, if oxygen was available the following reaction could compete ICl for the H2.
2H2(g)+O2(g)→2H2O(g)(4.3.9)
AIM Pharma 2023
-
 LIVE
LIVE
Badlands Media
6 hours agoBadlands Daily: September 26, 2025
3,950 watching -
 LIVE
LIVE
Matt Kohrs
12 hours agoPCE Inflation Report, BTFD & Payday Friday || Live Trading Stock Market Open
468 watching -
 LIVE
LIVE
Wendy Bell Radio
6 hours agoWelcome To The "Find Out" Phase
7,445 watching -
 LIVE
LIVE
GritsGG
1 hour agoQuad Win Streaks!🫡 Most Wins in WORLD! 3600+
91 watching -
 56:54
56:54
Crypto Power Hour
2 hours ago $0.58 earnedSpecial Guest Natalie Brunell, Author & Bitcoin Maxi
9.77K8 -
 LIVE
LIVE
Total Horse Channel
14 hours agoAMHA 2025 World Show 9/26
294 watching -
 LIVE
LIVE
LFA TV
18 hours agoBREAKING NEWS ALL DAY! | FRIDAY 9/26/25
3,088 watching -
 1:25:41
1:25:41
Chicks On The Right
5 hours agoComey's FAFO moment, Dallas sniper details, DFWYF, and who to trust in media.
32.4K12 -
 1:58:04
1:58:04
Welcome to the Rebellion Podcast
18 hours ago $1.93 earnedYou Made it to FriJay - WTTR Podcast Live 9/26
26K -
 1:29:14
1:29:14
Game On!
19 hours ago $2.70 earnedNFL Week 4 Betting Report Preview!
32K3