Premium Only Content
How to find percentage yield| theoretical yield| Actual yield|2020|AIM Pharma
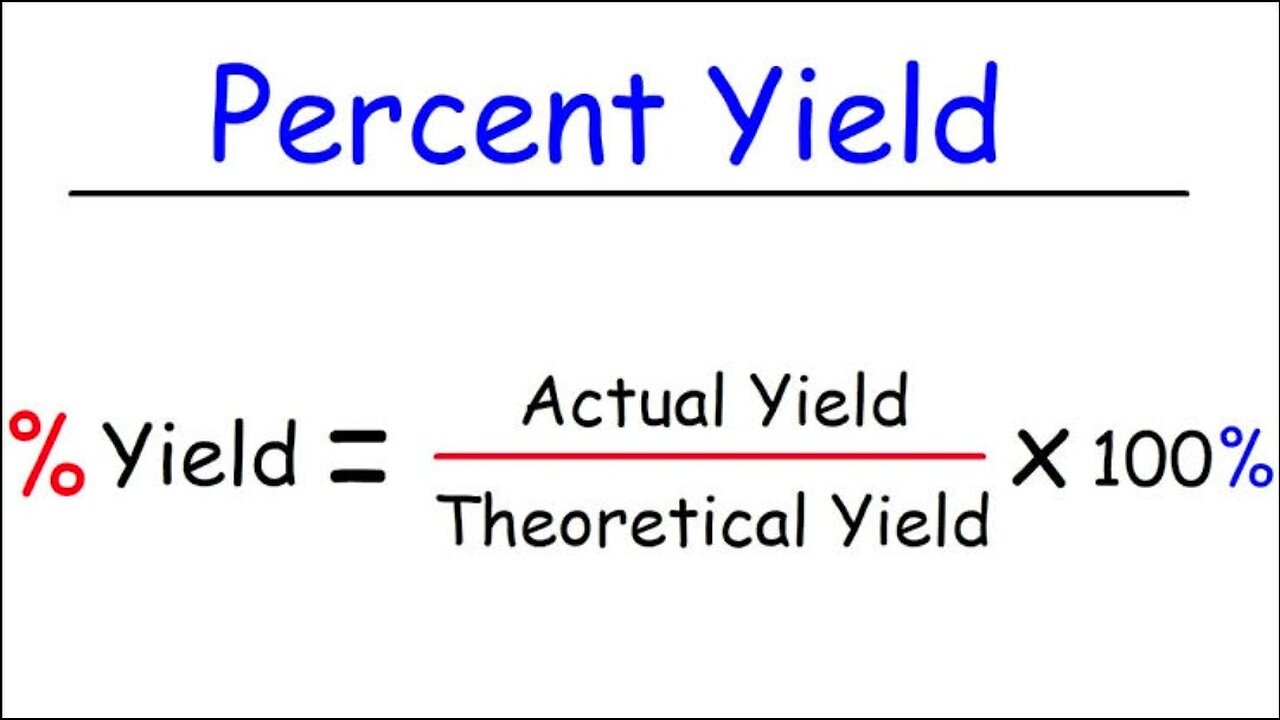
Percent Yield is defined as the actual yield divided by the theoretical yield times 100. Percent Yield=(Actual YieldTheoretical Yield)×100% There are many reasons why the actual yield of a chemical reaction may be less than the theoretical yield, and these will be taken up during later Chapters of the course.
Percent Yield
Percent Yield is defined as the actual yield divided by the theoretical yield times 100.
Percent Yield=(Actual YieldTheoretical Yield)×100%(4.3.1)
There are many reasons why the actual yield of a chemical reaction may be less than the theoretical yield, and these will be taken up during later Chapters of the course. Here are some reasons, most of which deal with topics that will be covered in the second semester of this course.
Equilibria between products and reactants, where the limiting reagent is not completely consumed (Chapter 15)
Ca3PO4(s)⇋3Ca+2+2PO−34(4.3.2)
Kinetics, simply speaking, the reaction may be very slow and not over (Chapter 14).
Formation of Intermediates. A chemical equation does not represent the mechanism but the overall balance of mass. The mechanism tells how the reaction actually proceeds (section 14.6), and there are often intermediate chemical species that are not completely consumed. This is exemplified in the following reaction.
H2(g)+2ICl(g)→I2(g)+2HClg(4.3.3)
It is highly unlikely that this reaction takes place in one step where three molecules simultaneously collide. A more plausible way for the reaction to occur is in two steps as below, where HI is an intermediate.
H2+IClHI+ICl→HCl+HI→HCl+I2(4.3.4)(4.3.5)
In the first step HCl and the intermediate HI are formed from the bimolecular collision of H2 and ICl. In the second step, the HI (intermediate) collides with another ICl to form HCl and I2. If you add the two steps the intermediate cancels out and you get the balanced equation.
H2(g)+2ICl(g)→I2(g)+2HClg(4.3.6)
4. Competing or Parallel Reactions
You may have a secondary reaction competing with the reaction described, like the formation of hydroiodic acid and chlorine gas.
H2+IClHCl+ICl→HI+HCl→HI+Cl2(4.3.7)(4.3.8)
So any HI or Cl2 formed would reduce the yield of the desired I2 and HCl. A parallel or competing reaction could also involve a completely different reactant. For example, if oxygen was available the following reaction could compete ICl for the H2.
2H2(g)+O2(g)→2H2O(g)(4.3.9)
AIM Pharma 2023
-
 1:07:26
1:07:26
Inverted World Live
12 hours agoThe War Against Robots w/ Joe Allen
94.5K5 -
 6:08:31
6:08:31
SpartakusLIVE
11 hours agoWARZONE NUKE IS BACK?! || Solo Challenge CHAMPION to start, duos w/ the Dawg later
97.6K1 -
 1:00:18
1:00:18
Man in America
13 hours agoBig Pharma’s Empire of Lies Is COLLAPSING as People Turn to Natural Medicine
60.9K20 -
 7:17:44
7:17:44
Drew Hernandez
15 hours agoGHISLAINE MAXWELL SAYS CLAIMS EPSTEIN WAS INTELLIGENCE ASSET ARE BULLSH*T?!
35K38 -
 29:54
29:54
Afshin Rattansi's Going Underground
23 hours agoUkraine: Prof. Anatol Lieven SLAMS Europe’s ‘BLOODY STUPIDITY’ as Trump Negotiates with Putin
33.1K6 -
 15:27
15:27
robbijan
1 day ago $2.46 earnedThe Emperor’s New Labubu & The Spiritual War Behind Everything
54K43 -
 LIVE
LIVE
GritsGG
21 hours ago36 Hour Stream! Most Wins 3420+ 🧠
813 watching -
 2:05:47
2:05:47
TimcastIRL
9 hours agoTrump FBI Raids John Bolton Amid Classified Docs Investigation | Timcast IRL
189K106 -
 2:15:23
2:15:23
TheSaltyCracker
9 hours agoFinally Someone Gets Raided ReEEeStream 8-22-25
93.4K240 -
 2:59:21
2:59:21
I_Came_With_Fire_Podcast
21 hours agoChina's New Ship Killers, EU Dead, Shooter HOAX, and The Missing Woman
20K3