Premium Only Content
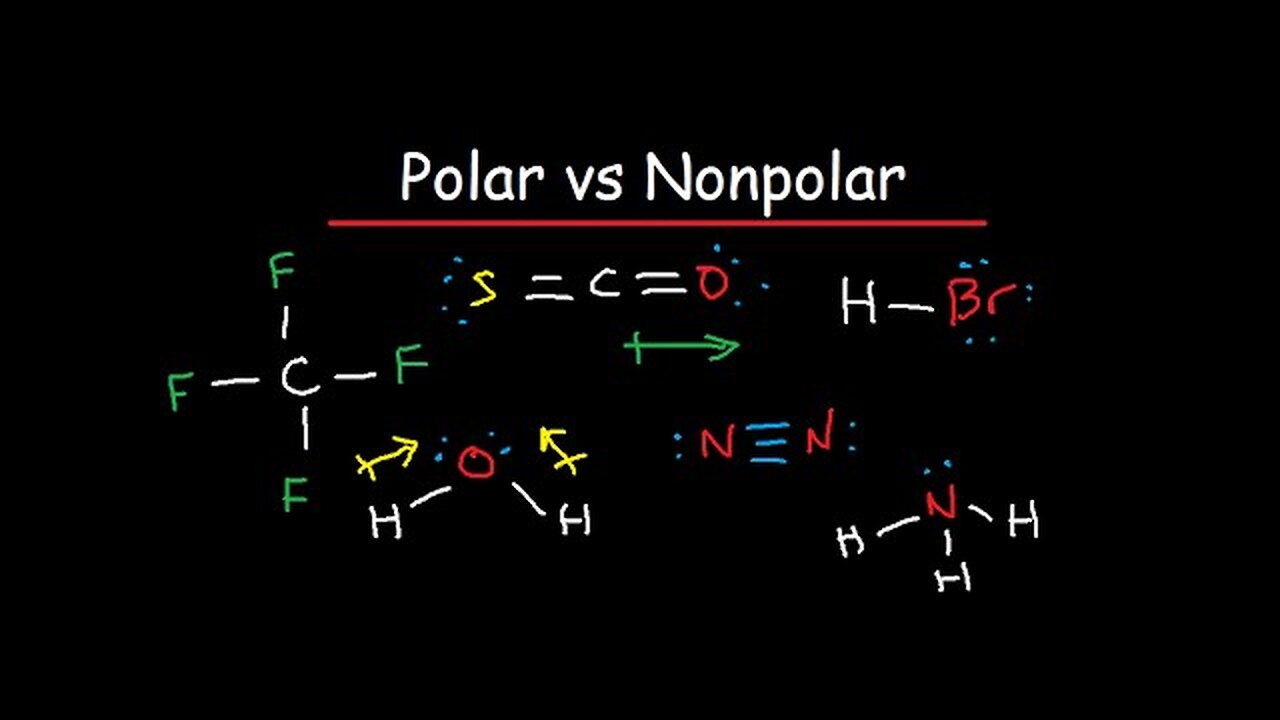
Polar and Nonpolar Molecules: Is it Polar or Nonpolar?
This video discusses how to tell if a molecule / compound is polar or nonpolar. Here is a list of molecules that are considered.
My E-Book: https://amzn.to/3B9c08z
Video Playlists: https://www.video-tutor.net
Homework Help: https://bit.ly/Find-A-Tutor
Subscribe: https://bit.ly/37WGgXl
Support & Donations: https://www.patreon.com/MathScienceTutor
Youtube Membership:
/ @theorganicchemistrytutor
General Chemistry Video Playlist:
• Intro to Chemistry, Basic Concepts - ...
Nonpolar Molecules:
Diatomic Molecules: H2, N2, O2, Cl2, Br2, F2, I2
Hydrocarbons: CH4, C2H6, C3H8, C2H2, C2H4
Identical Outer Elements With No Lone Pair on Central Atom:
Tetrahedral Molecular Geometry:
SiBr4, CCl4, CF4, GeH4, CBr4, SiH4
Trigonal Bipyramidal Molecular Geometry:
PCl5, PF5, AsF5, PBr5, SbCl5
Linear Molecular Geometry:
CO2, CS2, BeH2, BeCl2, and BeF2
Trigonal Planar Molecular Geometry:
BH3, AlCl3, AlBr3, AlF3, FeBr3
Octahedral Molecular Geometry:
SeF6, SBr6, SF6, SeCl6, SI6, SeI6
Polar Molecules:
Same Outer Element With an Assymetrical Lone Pair(s)
Bent Molecular Geometry:
H2S, H2O, H2Se, SF2, SCl2, SeBr2, SO2, SeO2
Trigonal Pyramidal Molecular Geometry:
NH3, PH3, PBr3, PCl3, NF3
T-shaped Molecular Geometry:
IF3, ClF3, BrF3, ICl3, BrCl3
Square Pyramidal Molecular Geometry:
IF5, ClF5, BrF5, ICl5, BrCl5
SeeSaw Molecular Geometry:
SF4, SeCl4, SBr4, SeI4
Exception: XeF4
Different Outer Elements: (Usually Polar)
CH3F, CSO, BH2F
Disclaimer: Some of the links associated with this video may generate affiliate commissions on my behalf. As an amazon associate, I earn from qualifying purchases that you may make through such affiliate links.
-
 11:02
11:02
TheOrganicChemistryTutor
10 months agoHow To Find The Square Root of Small Decimal Numbers Using Scientific Notation
1721 -
 19:32
19:32
MetatronHistory
21 hours agoWas Nazism Left Wing or Right Wing? An Answer From History
15.7K37 -
 DVR
DVR
Mally_Mouse
21 hours ago🌶️ 🥵Spicy BITE Saturday!! 🥵🌶️- Let's Play: Human Fall Flat
52.6K3 -
 4:36
4:36
GreenMan Studio
3 hours agoTHE RUMBLE COLLAB SHOW EP. 5 W/Greenman Reports
223 -
 LIVE
LIVE
ShitShow Gaming
4 hours agoOUTLAST 2 IN 2025. . . | #WWP #horrorgames #multistream
17 watching -
 DVR
DVR
Damysus Gaming
30 minutes agoARC Raiders - Grind to 75 is ON!! LFG!!!
-
 1:55:02
1:55:02
SavageJayGatsby
5 hours ago🔥 Spicy Saturday – Let's Play: Human Fall Flat🔥
16.4K2 -
 LIVE
LIVE
a12cat34dog
5 hours agoI'M FINALLY BACK :: Resident Evil 4 (2023) :: FINISHING MAIN GAME & DLC {18+}
200 watching -
 31:23
31:23
Stephen Gardner
4 hours agoFINALLY! Charlie Kirk MISSING DETAILS released!
26.2K145 -
 5:26:11
5:26:11
cosmicvandenim
9 hours agoCOSMIC VAN DENIM | SEX APPEAL & HORROR
20.6K10