Premium Only Content
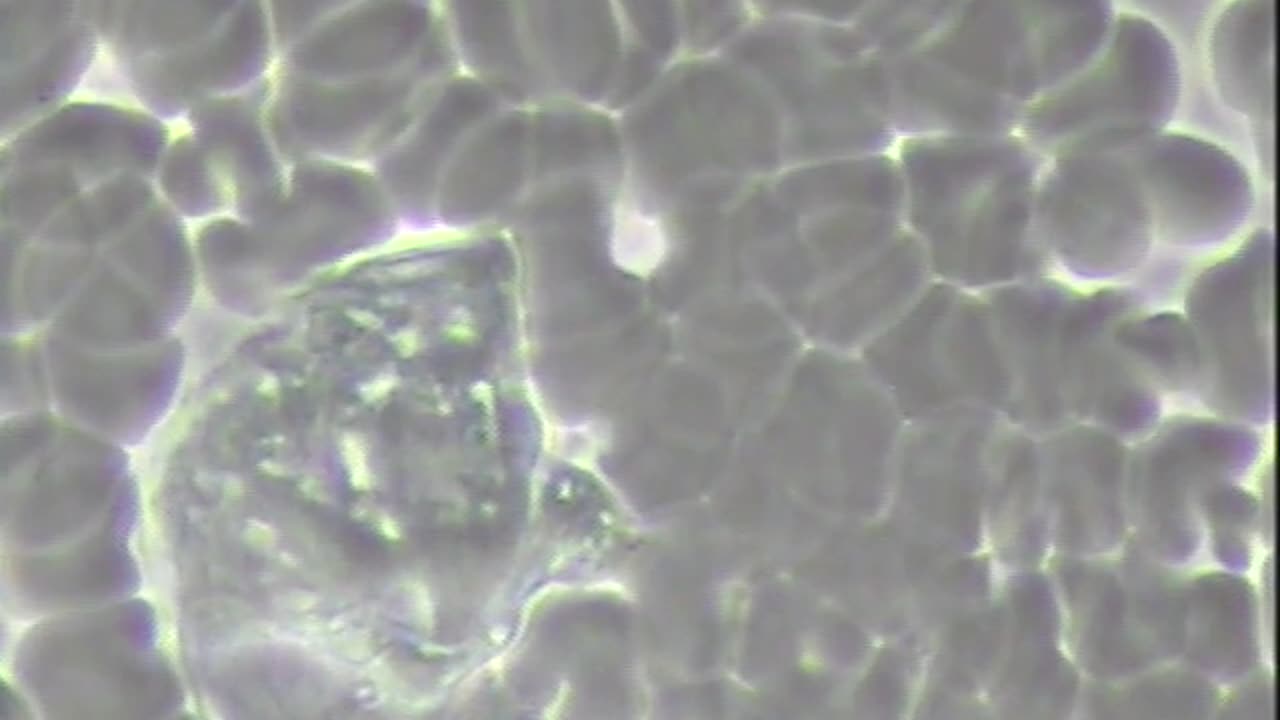
Diagnosed Inoperable Brain Cancer with METS to the Lung and Gall Bladder testing CoVid-19 +
The Blood Never Lies Notice the toxic polluted state of the internal environment of the vascular fluids created from a toxic acidic lifestyle, oil-based medicines, radiation and immunotherapy. This man was sent home to die after receiving 2 months of so-called cancer treatments and given only 3 weeks to live. He could not walk, eat, go to bathroom by himself or even speak. Because of a loving wife she helped her husband with a plant-based alkaline lifestyle and her husband is now talking, walking on his own, eating, sleeping, going to the bathroom on his own and has improved his changes for a better quality and quantity of life. Please watch and share this video and youtube channel with everyone you love and care about! www.drrobertyoung.com/blog SUMMARY CATALOGUE OF ERRORS FOUND IN THE CORMAN-DROSTEN PAPER CONCERNING RTPCR TESTING The Corman-Drosten paper contains the following specific errors: 1. There exists no specified reason to use these extremely high concentrations of primers in this protocol. The described concentrations lead to increased nonspecific bindings and PCR product amplifications, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 2. Six unspecified wobbly positions will introduce an enormous variability in the real world laboratory implementations of this test; the confusing nonspecific description in the Corman-Drosten paper is not suitable as a Standard Operational Protocol making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 3. The test cannot discriminate between the whole virus and viral fragments. Therefore, the test cannot be used as a diagnostic for intact (infectious) viruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus and make inferences about the presence of an infection. 4. A difference of 10° C with respect to the annealing temperature Tm for primer pair1 (RdRp_SARSr_F and RdRp_SARSr_R) also makes the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 5. A severe error is the omission of a Ct value at which a sample is considered positive and negative. This Ct value is also not found in follow-up submissions making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 6. The PCR products have not been validated at the molecular level. This fact makes the protocol useless as a specific diagnostic tool to identify the SARS-CoV-2 virus. 7. The PCR test contains neither a unique positive control to evaluate its specificity for SARS-CoV-2 nor a negative control to exclude the presence of other coronaviruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 8. The test design in the Corman-Drosten paper is so vague and flawed that one can go in dozens of different directions; nothing is standardized and there is no SOP. This highly questions the scientific validity of the test and makes it unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 9. Most likely, the Corman-Drosten paper was not peer-reviewed making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus. 10. We find severe conflicts of interest for at least four authors, in addition to the fact that two of the authors of the Corman-Drosten paper (Christian Drosten and Chantal Reusken) are members of the editorial board of Eurosurveillance. A conflict of interest was added on July 29 2020 (Olfert Landt is CEO of TIB-Molbiol; Marco Kaiser is senior researcher at GenExpress and serves as scientific advisor for TIB-Molbiol), that was not declared in the original version (and still is missing in the PubMed version); TIB-Molbiol is the company which was “the first” to produce PCR kits (Light Mix) based on the protocol published in the Corman-Drosten manuscript, and according to their own words, they distributed these PCR-test kits before the publication was even submitted;[1] further, Victor Corman & Christian Drosten failed to mention their second affiliation: the commercial test laboratory “Labor Berlin”. Both are responsible for the virus diagnostics there [2] and the company operates in the realm of real time PCR-testing. In light of our re-examination of the test protocol to identify SARS-CoV-2 described in the Corman-Drosten paper we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless. [1] Instructions For Use LightMix SarbecoV E-gene plus EAV Control, TIB-Molbiol & Roche Molecular Solutions, January 11th 2020: https://www.roche-as.es/lm_pdf/MDx_40... gene_V200204_09164154001 (1).pdf Archive, timestamp – January 11th 2020: https://archive.is/Vulo5; Archive: https://bit.ly/3fm9bXH [2] Christian Drosten & Victor Corman, responsible for viral diagnostics at Labor Berlin: https://www.laborberlin.com/fachberei... https://archive.is/CDEUG
Source: https://www.youtube.com/watch?v=E2JjZffQYLQ
20210220
-
 7:37
7:37
Dr Robert O. Young Videos (Unofficial)
1 year agoSolutions for Electronic Warfare by Sabrina Wallace
4.8K5 -
 LIVE
LIVE
Barry Cunningham
3 hours agoPRESIDENT TRUMP TOURS JEROME POWELL SCENE OF THE CRIME AT THE FEDERAL RESERVE
1,812 watching -
 1:25:14
1:25:14
Sarah Westall
2 hours agoBlackmail, Power & Corruption: The Currency of the Empire - Epstein & more w/ Joachim Hagopian
5.75K4 -
 16:33
16:33
Clownfish TV
7 hours agoLate Night TV Has NO FUTURE After Stephen Colbert's Cancellation!
7517 -
 45:56
45:56
The White House
3 hours agoPresident Trump Visits the Federal Reserve
19.2K19 -
 36:14
36:14
Kimberly Guilfoyle
4 hours agoRussia Hoax Reality Check, Live! | Ep240
17.5K10 -
 11:02
11:02
Preston Stewart
7 hours ago $0.18 earnedThailand–Cambodia Clash Erupts
3.5K3 -
 LIVE
LIVE
LFA TV
21 hours agoLFA TV ALL DAY STREAM - THURSDAY 7/24/25
1,063 watching -
 1:07:35
1:07:35
vivafrei
4 hours agoWall Street Journal DOUBLING DOWN on Epstein! Lawyer in Canada DEBANKED! AND MORE!
99.6K33 -
 4:42:20
4:42:20
Donut Operator
7 hours agoI'M BACK/ CRIME/ GAMEBOY CAMERA CHAD
100K5