Premium Only Content
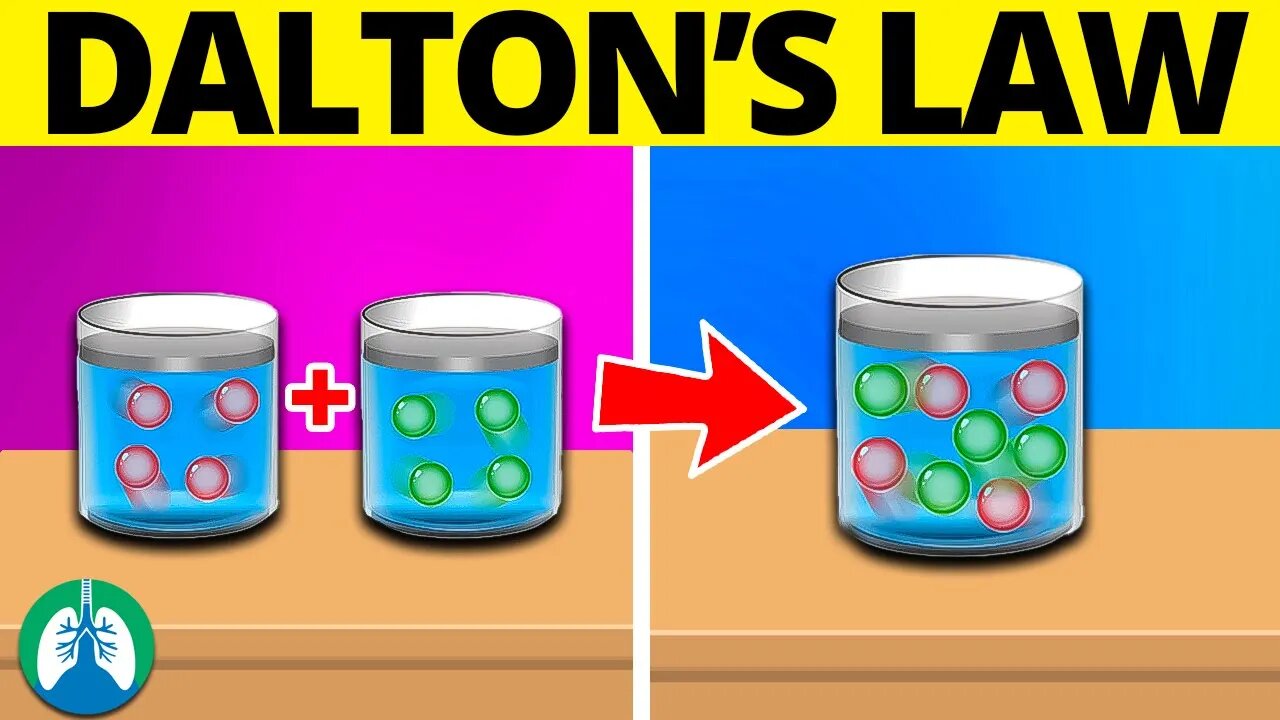
Dalton's Law (Medical Definition) | Quick Explainer Video
What is Dalton's law? This video covers the medical definition and provides a brief overview of this topic. Watch to learn more!
💥Respiratory Therapy Definitions [Glossary] ➜ ➜ ➜ https://bit.ly/3g6s4Pj
➡️ Dalton's Law Formula:
Ptotal = P1 + P2 + P3 + ...
The partial pressure of a gas is the pressure that it would exert if it were present alone at the same volume and temperature as the mixture. This principle is based on the kinetic molecular theory, which states that gases are made up of rapidly moving particles that are in constant motion. These particles collide with each other and the walls of their container, exerting pressure on the container. In a mixture of gases, each gas exerts its own pressure on the container, and the total pressure of the mixture is simply the sum of the pressures of each individual gas.
➡️ Application
One of the most important applications of Dalton's Law is in the field of respiratory physiology, where it is used to explain the behavior of gases in the lungs. The air we breathe is a mixture of different gases, primarily nitrogen, oxygen, and carbon dioxide. Each of these gases exerts its own pressure in the lungs, and the total pressure of the air we breathe is equal to the sum of the pressures of each individual gas. This principle is also used in the design and operation of breathing devices used by scuba divers and astronauts, as well as in the study of atmospheric gases.
➡️ Dalton's Law
It's also used in industrial settings, particularly in the production and storage of gases. Therefore, understanding the behavior of gases in a mixture is crucial for the safe and efficient operation of gas storage tanks, pipelines, and other equipment. Additionally, the law is useful in the study of combustion and explosions, as it can help to determine the flammability limits of various gas mixtures.
➡️ Principle
Dalton's Law is a fundamental principle in the study of gases and their behavior. It states that the total pressure of a mixture of gases is equal to the sum of the pressures of each individual gas in the mixture. This principle has wide-ranging applications in a variety of fields, including respiratory physiology, atmospheric science, and industrial chemistry, and is a crucial concept for anyone studying or working with gases.
💥Respiratory Therapy Definitions [Glossary] ➜ ➜ ➜ https://bit.ly/3g6s4Pj
—————
📗 BEST STUDY GUIDES FOR YOU
▪ TMC Test Bank 👉 http://bit.ly/2IGeqSu
▪ Hacking the TMC Exam 👉 http://bit.ly/2XBc8do
▪ TMC Exam Bundle (Save $) 👉 https://bit.ly/34pqEsV
▪ Daily TMC Practice Questions 👉 http://bit.ly/2NnXh3C
💙MORE FROM RTZ
▪ Free TMC Practice Exam 👉 http://bit.ly/2XlwASL
▪ Free RRT Cheat Sheet 👉 http://bit.ly/2IbmOKB
▪ Resources for RT's 👉 http://bit.ly/2WVV5qo
▪ Testimonials 👉 http://bit.ly/2x7b5Gl
🌐FOLLOW US
▪ Instagram 👉 http://bit.ly/2FhF0jV
▪ Twitter 👉 http://bit.ly/2ZsS6T1
▪ Facebook 👉 http://bit.ly/2MSEejt
▪ Pinterest 👉 http://bit.ly/2ZwVLPw
🚑MEDICAL DISCLAIMER
This content is for educational and informational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Please consult with a physician with any questions that you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you watch in this video. We strive for 100% accuracy, but errors may occur, and medications, protocols, and treatment methods may change over time.
💡AFFILIATE DISCLAIMER
This description contains affiliate links. If you decide to purchase a product through one of them, we receive a small commission at no cost to you.
—————
⏰TIMESTAMPS
0:00 - Intro
0:58 - Formula
1:55 - Respiratory Physiology
2:30 - Industrial Setting
3:00 - Study of Gases
—————
🖼CREDIT FOR MUSIC AND GRAPHICS:
▪ Music licensed from Audiojungle.net/
▪ Graphics: Canva.com, Freevector.com, Vecteezy.com, and Pngtree.com
#RespiratoryTherapy #RespiratoryTherapist #DaltonsLaw
-
 4:01
4:01
Respiratory Therapy Zone
1 year agoLung Lobes and Fissures *EXPLAINED* 🫁
460 -
 1:54:43
1:54:43
Side Scrollers Podcast
16 hours agoVoice Actor ROASTED For Racist Double Standard + Influencer FELONY After Con Threat | Side Scrollers
21.4K7 -
 11:03
11:03
The Pascal Show
16 hours ago'THAT BABY IS GONE!' Neighbors Break Silence On Emmanuel Haro Case... Wildlife Took Remains?!
1.71K -
 LIVE
LIVE
Lofi Girl
2 years agoSynthwave Radio 🌌 - beats to chill/game to
284 watching -
 2:54:09
2:54:09
Badlands Media
13 hours agoDEFCON ZERQ Ep. 007: Flynn, Q Signals, and the Venezuela Strike
111K46 -
 2:29:05
2:29:05
FreshandFit
4 hours agoOwen Shroyer leaves Alex Jones But Here's the Truth!
18.6K15 -
 2:04:10
2:04:10
Inverted World Live
9 hours agoJapanese Memory Eraser | Ep. 101
101K18 -
 2:42:52
2:42:52
TimcastIRL
7 hours agoTrump To Deploy National Guard To Chicago, Baltimore, Democrats Call To Resist | Timcast IRL
223K92 -
 3:22:30
3:22:30
Laura Loomer
8 hours agoEP141: Muslims Call For Political Assassinations At Michigan Palestinian Conference
52.2K27 -
 4:39:32
4:39:32
Barry Cunningham
9 hours agoBREAKING NEWS: PRESIDENT TRUMP IS GOING TO TAKE CHICAGO! LFG!!! (IT'S MOVIE NIGHT!)
95.1K65