Premium Only Content
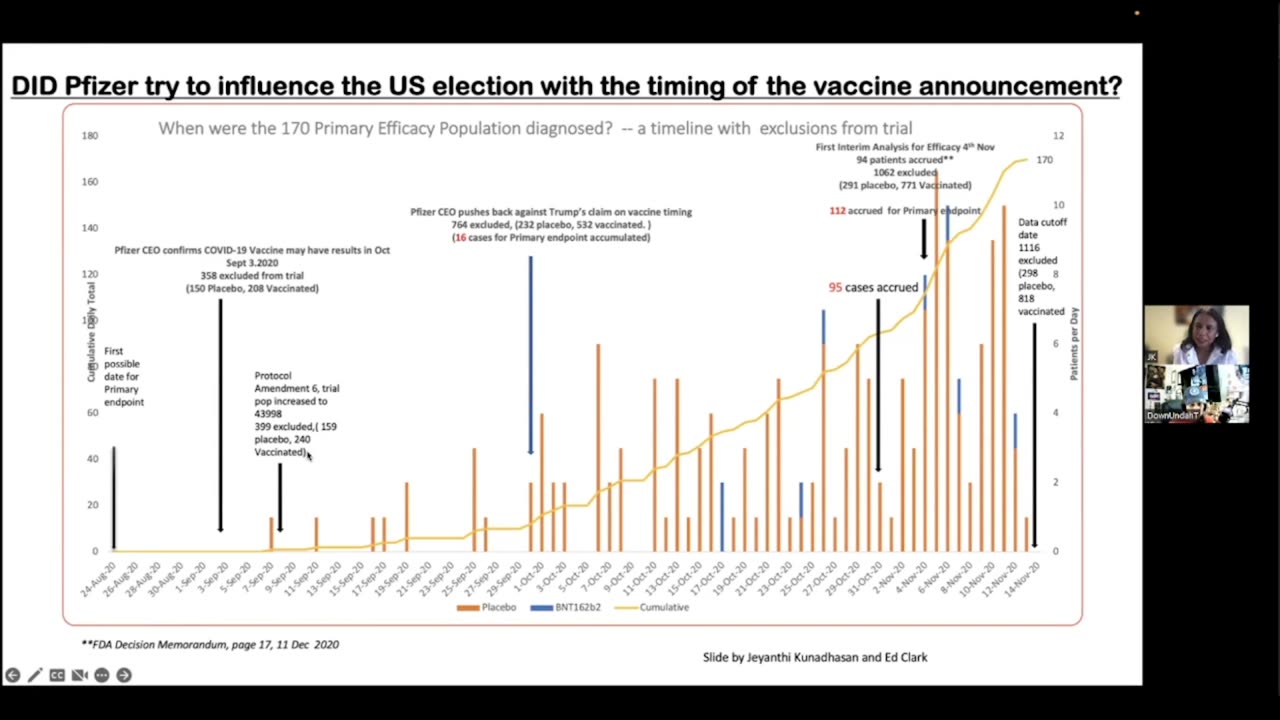
Did Pfizer Try to Influence the 2020 US Election With the Timing of the Vaccine Announcement?
Dr. Jeyanthi Kunadhasan breaks down the timeline.
According to contracts with the Trump Administration, Pfizer was supposed to deliver an effective vaccine by October 31.
However, it didn't play out that way. And when the data cutoff date came, proportionately more vaccinated people were being excluded from the trial than the placebo group.
The clinical trial timeline unrolled as follows:
July 27- Nov 14, 2020: The clinical trial run by Pfizer to determine efficacy enrolled approximately 44,000 participants.
Nov 20: Pfizer submitted their data with a request for an Emergency Use Authorization (EUA) for its COVID vaccine
Dec 10, 2020: FDA held a meeting to discuss Pfizer’s EUA request
Dec 11, 2020: EUA was APPROVED
Dec 14, 2020: First dose administered to the public
• Full Video: https://rumble.com/v2cwsy4-mmitb-ep.-38-dr.-jeyanthi-kunadhasan.html
• Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA: https://dailyclout.io/report-41-the-170-clinical-trial-participants-who-changed-the-world-pfizer-ignored-protocol-deviations-to-obtain-emergency-use-authorization-for-its-covid-19-mrna-vaccine/
• Pfizer Broke Its Own Rules to Obtain EUA: https://dailyclout.io/war-room-dailyclout-research-team-finds-serious-protocol-deviations-among-the-170-patients-that-the-covid-vaccine-eua-is-based-on/
-
 35:10
35:10
Vigilant Fox
4 months agoTranshumanist Code: AI Psychosis Crisis | Daily Pulse Ep 60
10.7K14 -
 2:00:53
2:00:53
Tundra Tactical
13 hours ago $0.89 earned🛑LIVE AT 9PM CST!! Your Government Hates Your Guns : DOJ Holds Firm On National FIREARMS ACT
13.3K -
 LIVE
LIVE
DLDAfterDark
3 hours ago $1.16 earnedAre YOU The Guy That Ruins Thanksgiving?? - God Guns & Gear
341 watching -
 2:58:31
2:58:31
NewsTreason
5 hours agoDECLAS w/ Rambo & Dave: Nuremberg 2.0 | MTG Exits Stage Left | Mamdani Psyop Confirmed, 8pm EST
61.8K61 -
 LIVE
LIVE
meleegames
4 hours agoSONG REQUESTS CLOSED - Melee Music - Beat Hazard 3 - Devil Inside
180 watching -
 2:13:31
2:13:31
The Connect: With Johnny Mitchell
12 hours ago $1.62 earnedIs Garth Brooks A Serial Killer? Exposing The Dark Secrets Of Country Music's Biggest Star
10.8K3 -
 1:00:49
1:00:49
MattMorseTV
5 hours ago $79.23 earned🔴Massive VICTORY in the SUPREME COURT.🔴
100K129 -
 4:08:14
4:08:14
GritsGG
4 hours ago#1 Most Warzone Wins 4015+!
12.7K -
 4:20:08
4:20:08
Biscotti-B23
7 hours ago $1.06 earned🔴 LIVE STREAM ENDS WHEN I GET 100 WINS 🥵 INVINCIBLE VS CLOSED ALPHA
11.4K3 -
 12:38
12:38
Timcast
1 day agoJasmine Crocket HUMILIATED By CNN To HER FACE Over Epstein LIE | Tim Pool
111K77