Premium Only Content
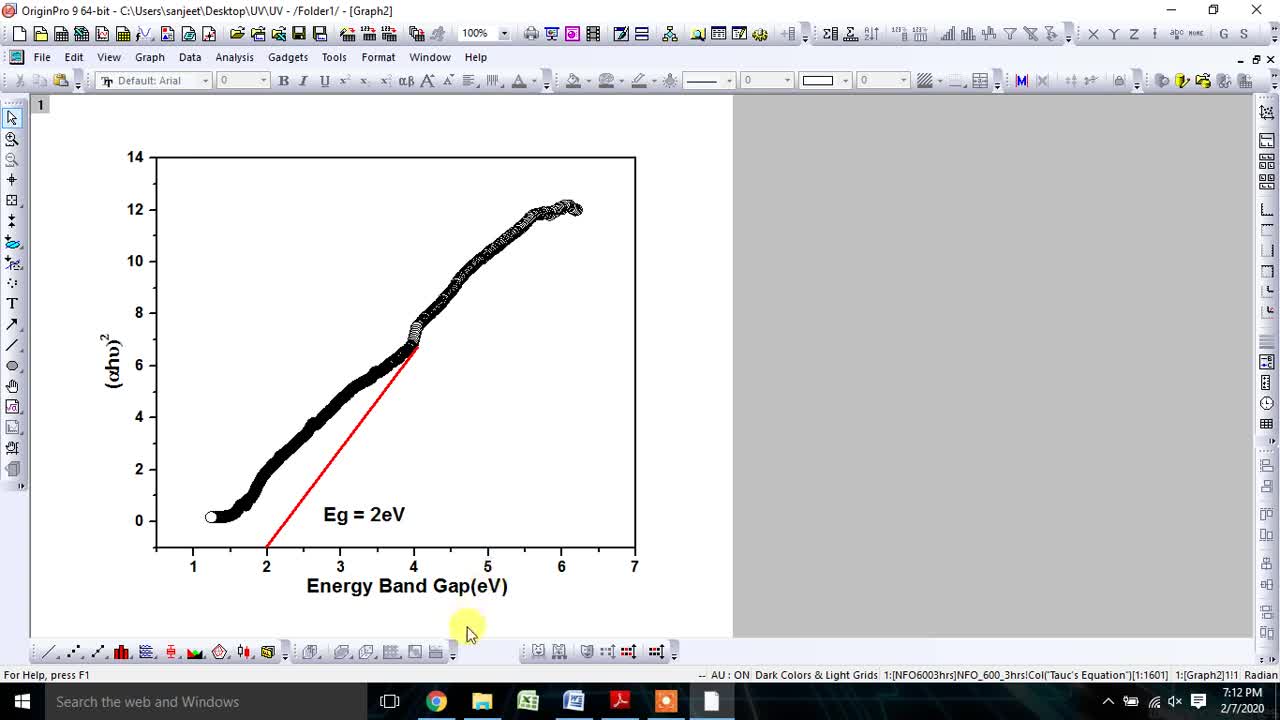
Estimate the energy band gap using Tauc's Equation from UV-vis spectroscopy data via origin soft.
UV-Vis spectroscopy is an analytical technique that measures the amount of discrete wavelengths of UV or visible light absorbed by or transmitted through a sample in comparison to a reference or blank sample. This property is influenced by sample composition, potentially providing information on what is in the sample and at what concentration. Because this spectroscopy technique relies on the use of light, we first considered the properties of light.
Light has a certain amount of energy that is inversely proportional to its wavelength. Thus, shorter wavelengths of light carry more energy and longer wavelengths carry less energy. A specific amount of energy is required to promote electrons in a substance to a higher energy state, which can be detected as absorption. Electrons in different bonding environments in a substance require a specific amount of energy to promote the electrons to a higher energy state. This is why light absorption occurs at different wavelengths for different substances. Humans are able to see a spectrum of visible light, from approximately 380 nm, which we see as violet, to 780 nm, which we see as red.1 UV light has wavelengths shorter than that of visible light to approximately 100 nm. Therefore, light can be described by its wavelength, which can be useful in UV-Vis spectroscopy to analyze or identify different substances by locating the specific wavelengths corresponding to the maximum absorbance (see the Applications of UV-Vis spectroscopy section).
-
 1:25:34
1:25:34
Kim Iversen
1 hour agoEpstein to Empire: Scott Horton Unpacks the Lies That Led Us Here
69.8K24 -
 1:48:16
1:48:16
Redacted News
2 hours agoSomething BIG is happening in Ukraine, new CIA coup? Putin launches new massive offensive | Redacted
131K28 -
 53:22
53:22
Candace Show Podcast
3 hours agoBREAKING! Brigitte Sues Me For Defamation | Candace Ep 218
44.2K73 -
 LIVE
LIVE
Robert Gouveia
1 hour agoObama CRIMINAL REFERRAL!! Tulsi Drops MOTHERLODE! Senators DEMAND Charges!
1,573 watching -
 LIVE
LIVE
Dr Disrespect
7 hours ago🔴LIVE - DR DISRESPECT - 10 WINS CHALLENGE - BIG ANNOUNCEMENT AT 12PM PT
1,954 watching -
 LIVE
LIVE
Barry Cunningham
2 hours agoPRESIDENT TRUMP MAKES SPEECH ABOUT AI
1,311 watching -
 LIVE
LIVE
The Jimmy Dore Show
52 minutes agoJon Stewart LIVID About Colbert Show Cancelation! Trump ADMITS His Name Is in the Epstein Files!
4,549 watching -
 13:18
13:18
The Rad Factory
8 hours ago $0.16 earnedIs This E Scooter Worth $900?
3.56K -
 54:38
54:38
The Dr. Ardis Show
7 hours ago $1.76 earnedThe Dr. Ardis Show | 5 Ways Alcohol Destroys Your Health | Episode 07.23.2025
4.47K5 -
 LIVE
LIVE
RalliedLIVE
5 hours ago $1.47 earned10 WINS WITH THE SHOTTY BOYS
130 watching