Premium Only Content
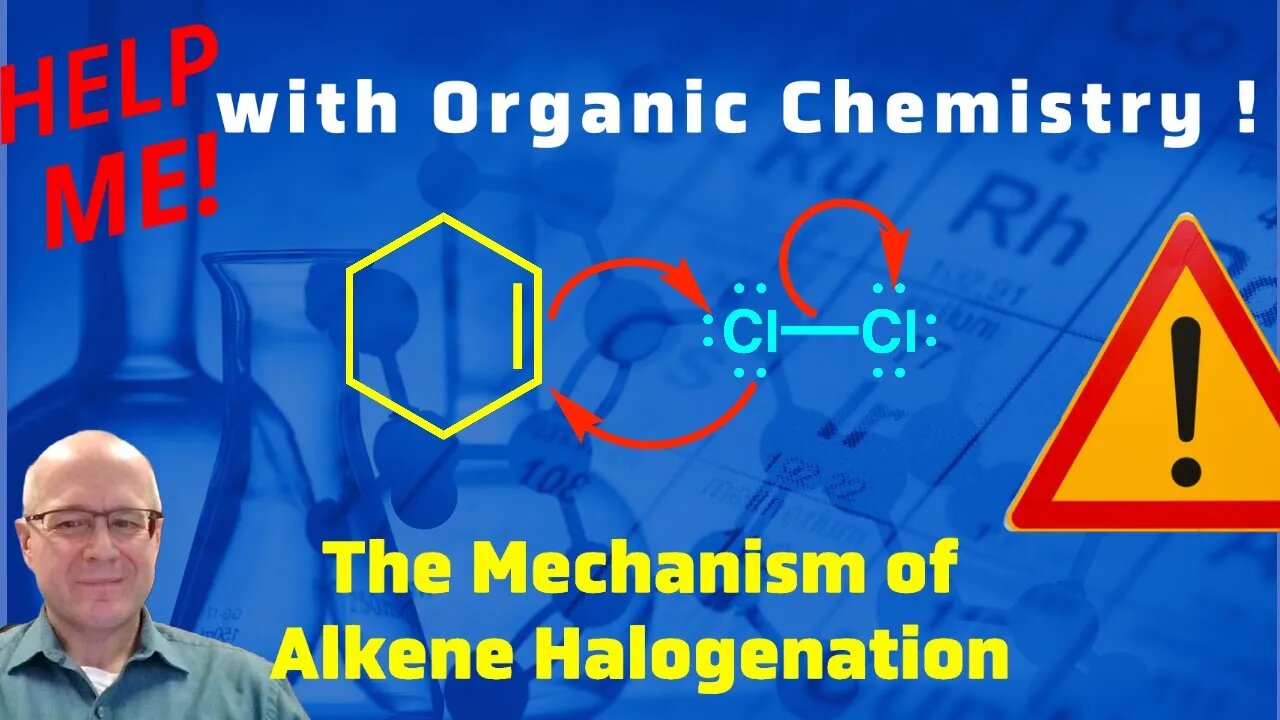
What is the Mechanism of the Halogenation of an Alkene? Help Me With Organic Chemistry
This video will discuss the mechanism of the halogenation of an alkene. The addition of a halogen to an alkene. The mechanism of halogenation is a key mechanism you must understand in organic chemistry. To start the alkene attacks that haolgen (chlorine, bromine) and the leaving group (the other halogen) leaves. At the same time a lone pair of the halogen attacks the carbon of the alkene. All the bond forming and bond breaking reactions occur at the same time making this another example of a concerted reaction. This mechanism goes through an ion call the halonium ion (chloronium, bromonium). The halonium ion is then attacked by the halide that was liberated in the first step (chloride, bromide) to form the dihalide.
Failing organic chemistry? You do not have to fail Organic Chemistry!
This video is part of a series called How to be Successful in Organic Chemistry. In this series I go over numerous problems that a student could expect to see in there organic chemistry 1 course. Doing organic chemistry practice problems will make you more successful in organic chemistry and biochemistry.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
-
 5:58
5:58
Chemistry Tutor
2 years ago $0.02 earnedHow to Draw a Lewis Structure From a Molecular Formula C6H14O2 Help with Chemistry!
138 -
 17:14
17:14
Mrgunsngear
2 hours ago $1.73 earnedUpdate: Current Glocks Discontinued & Glock V Series Is Coming!
1.26K15 -

Barry Cunningham
2 hours agoMUST SEE: PRESIDENT TRUMP NATO PRESSER! AND NEW YORK CITY MAYORAL DEBATE!
18.6K32 -
 13:15
13:15
Cash Jordan
5 hours ago"INVASION" Mob STRIKES Chicago Jail… FRONTLINE Marines IGNORE Judge, SMASH Illegals
2.34K9 -
 LIVE
LIVE
SpartakusLIVE
2 hours ago#1 Solo Challenge CHAMPION entertains HERDS of NERDS
135 watching -
 LIVE
LIVE
Alex Zedra
57 minutes agoLIVE! New Game | DeathWatchers
82 watching -
 LIVE
LIVE
Nikko Ortiz
2 hours agoShotguns With A Magazine... |Rumble Live
55 watching -
 23:18
23:18
Lady Decade
6 hours agoThe Diversity Lie Gaming Refuses To Talk About
1.1K4 -
 LIVE
LIVE
Geeks + Gamers
1 hour agoGeeks+Gamers Play- MARIO KART WORLD
136 watching -
 LIVE
LIVE
Midnight In The Mountains™
4 hours agoGaming w/ Midnight | Studio is BACK and SO ARE WE | 3 AWAY FROM 1,500 WILL YOU GET ME THERE?!
36 watching