Premium Only Content
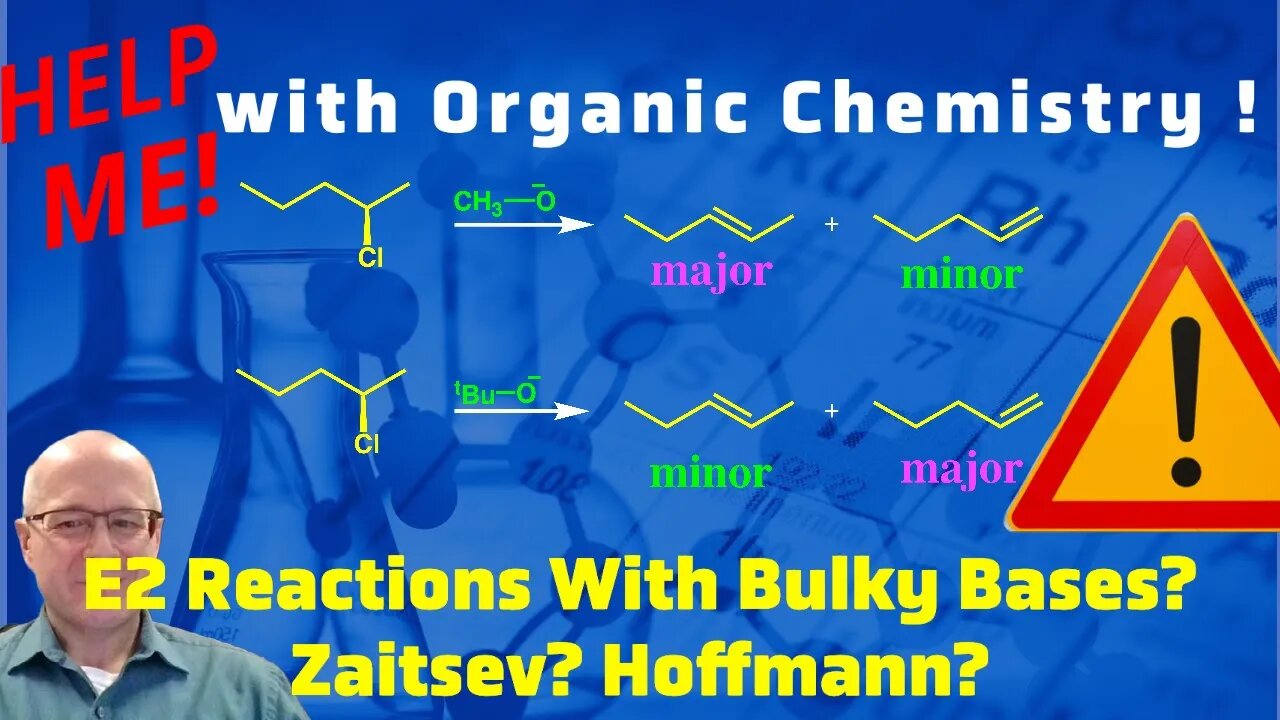
E2 Reactions and Bulky Bases Hoffmann vs Zaitsev Products in E2 Reactions. Help me with Org. Chem!
The E2 reaction delivers alkene products. A reaction that follows an E2 mechanism becomes interesting when two different alkenes can be formed depending on the size of the base employed. In deed, an alkene that is the most substituted is called the Zaitsev product and is generally the most stable alkene to form. The Hoffmann product is the alkene that is the least substituted and is generally less stable than the more substituted alkene. If a chemist wanted to produce the more substituted alkene as the major product they should use a small (non bulky) base. If a chemist wanted to produce the Hoffmann product they should use a large (bulky) base.
The E2 mechanism is second order. This means that the base and the substrate exist together in the transition state of the rate determining step.
Failing organic chemistry? You do not have to fail Organic Chemistry!
This video is part of a series called How to be Successful in Organic Chemistry. In this series I go over numerous problems that a student could expect to see in there organic chemistry 1 course. Doing organic chemistry practice problems will make you more successful in organic chemistry and biochemistry.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
https://www.dropbox.com/s/65hbm3a4w0p2epl/hoffmann%20versus%20zaitsev%20e2%20reaction.key?dl=0
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
-
 3:21
3:21
Chemistry Tutor
2 years ago $0.04 earnedNursing School Math Problems: Milliters of Amoxicillin per Day to Total Milligrams of Amoxicillin
156 -
 LIVE
LIVE
Barry Cunningham
6 hours agoBREAKING NEWS: PRESIDENT TRUMP SET TO TAKEOVER CHICAGO AND BOSTON!
2,623 watching -
 LIVE
LIVE
SpartakusLIVE
51 minutes agoThe BADDEST Duo in WZ Exhibits PEAK Physique || Duos w/ Sophiesnazz to start, quads later
835 watching -
 LIVE
LIVE
LumpyPotatoX2
1 hour agoSunday Funday on HellDivers - #RumbleGaming
83 watching -
 3:11:52
3:11:52
Esports Awards
3 hours agoEsports Awards: Decade Awards 2025
29K3 -
 1:02:58
1:02:58
Sarah Westall
2 hours agoMILITARY WHISTLEBLOWER: How Social Media Military Level Psyops are Manipulating You w/ Patrick Bergy
6261 -
 30:41
30:41
Stephen Gardner
1 hour ago🔥WHITE HOUSE GETS UNEXPECTED BIG WIN!
1.13K9 -
 9:39
9:39
MattMorseTV
3 hours ago $0.31 earnedVance just DROPPED a BOMBSHELL.
3.06K36 -
 2:40:14
2:40:14
DooM49
3 hours agoThe Grind for Battlefield 6 Skins - A-10 Unlocked
29 -
 1:47:49
1:47:49
Jeff Ahern
3 hours ago $0.23 earnedThe Sunday Show!
111K4