Premium Only Content
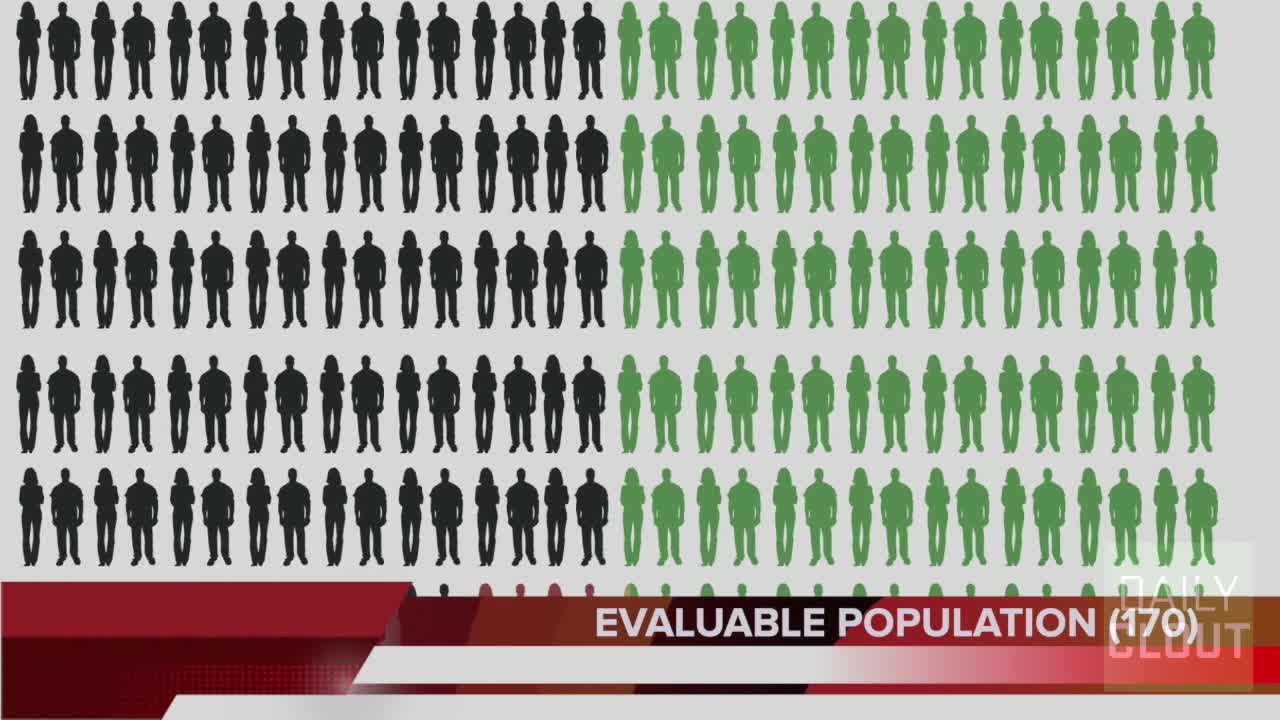
How Pfizer's EUA Was Granted Based on Less Than 0.4% of Clinical Trial Participants
1. The pivotal Pfizer trial upon which the Emergency Use Authorization (EUA) for the COVID -19 vaccines was on the endpoint of efficacy of the vaccines.
2. This was to be done when the number of eligible patients ("evaluable efficacy population") reached a threshold for analysis. The threshold was 164 trial participants.
3. We show that this threshold was likely not met and that the granting of the EUA should revisited.
4. There seemed to be a deliberate ambiguity left in the trial protocol documents to allow patients who would otherwise be deemed to have violated the trial protocol to still be included in the analysis. There was marked difference in what was implicitly stated to be the appropriate dosing interval between Dose 1 and Dose 2 in the trial protocol and all subsequent 14 protocol amendments, and what was accepted and approved in the EUA memorandum in December 2020.
5. We also show that patients in the final efficacy analysis had other significant protocol deviations, besides just going outside of the protocol's dosing interval.
-
 25:43
25:43
Russell Brand
1 day agoThis Is Getting Out Of Hand
126K127 -
 LIVE
LIVE
The Quartering
14 hours agoThanksgiving Day Yule Log!
1,727 watching -
 15:32
15:32
IsaacButterfield
22 hours ago $3.96 earnedAussie Reacts To UNHINGED Woke TikToks!
20.5K10 -
 3:24:28
3:24:28
PandaSub2000
14 hours agoNintendo Platformers - Thanksgiving 2025 Special | ULTRA BEST AT GAMES (Original Live Version)
41.5K8 -
 1:03:06
1:03:06
MetatronGaming
1 day agoThis is the scariest game ever (for an Italian)
28.9K10 -
 1:09:35
1:09:35
The White House
9 hours agoPresident Trump Participates in a Call with Service Members
50K86 -
 5:20:01
5:20:01
a12cat34dog
8 hours agoHAPPY THANKSGIVING - I APPRECIATE YOU ALL SO MUCH {18+}
25.1K4 -
 24:55
24:55
Jasmin Laine
1 day agoCarney BRAGS About ‘Investment’—Poilievre Drops a FACT That Stops the Room
32.2K29 -
 2:14:15
2:14:15
SIM_N_SHIFT GAMING
7 hours ago $1.64 earnedGRAND THEFT AUTO WITH FRIENDS
16.6K -
 6:43:27
6:43:27
VikingNilsen
17 hours ago🔴LIVE - VIKINGNILSEN - THE NEW PRELUDE - SOULFRAME
14.9K