Premium Only Content
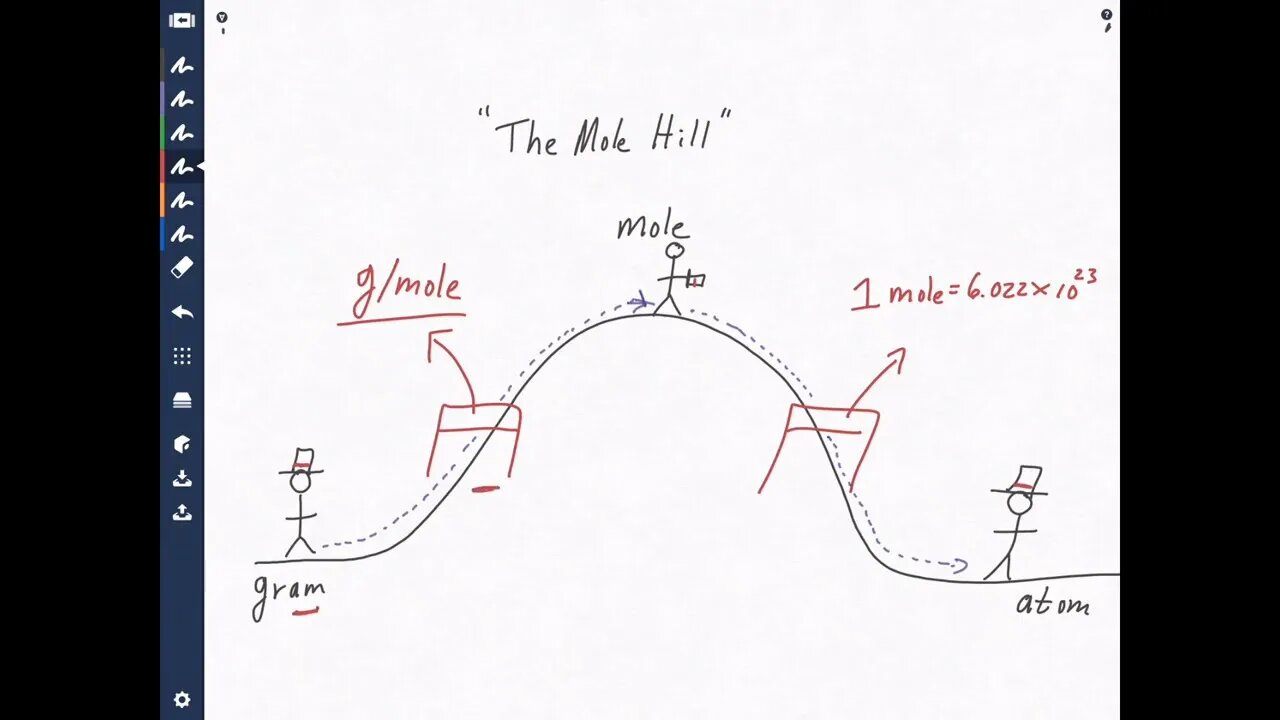
Converting Grams to Moles, Moles to Atoms, Grams to Atoms Avogadro's number
Converting grams to moles, moles to atoms and grams to atoms is fundamental. In fact you will see this on chemistry exams! This video will teach you how to convert grams to moles, how to convert moles to atoms and how to convert grams to atoms. Converting grams to atoms (g to atoms), moles to atoms and grams to moles (g to moles) is easy if you use dimensional analysis and Avogadro's number (6.022 x 10^23). Avogadro's number is often referred to as Avogadro’s Constant. The Avogadro's number definition is 6.022 x 10^23.
Please like this video and leave a comment below! It really helps and is so encouraging.
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
#convertinggramstomoles
#convertmolestograms
#gramstomolesconversion
#convertinggramstoatoms
#convertingmolestoatoms
#convertgtomoles
#avogadroconstant
#avogadrosnumber
#avogadro
#avogadrosnumber
-
 5:58
5:58
Chemistry Tutor
1 year ago $0.02 earnedHow to Draw a Lewis Structure From a Molecular Formula C6H14O2 Help with Chemistry!
86 -
 2:21:35
2:21:35
Price of Reason
13 hours agoThe Establishment WORRIES about Elon Musk AGAIN! Superman Trailer Discussion! Sonic 3 Review!
45.1K3 -
 1:14:54
1:14:54
Steve-O's Wild Ride! Podcast
17 hours ago $19.97 earnedZac Brown Reveals The Secrets To HIs Success - Wild Ride #247
76.2K15 -
 4:16:43
4:16:43
JdaDelete
11 hours ago $12.93 earnedProject Zomboid with the Boys | The Great Boner Jam of 2025
47.4K -
 6:00:08
6:00:08
SpartakusLIVE
11 hours agoYoung Spartan STUD teams with old gamers for ultimate BANTER with a SMATTERING of TOXICITY
35.8K -
 1:50:39
1:50:39
Kim Iversen
13 hours agoShocking Proposal: Elon Musk for Speaker of the House?! | IDF Soldiers Reveal Atrocities—'Everyone Is a Terrorist'
82.2K189 -
 43:27
43:27
barstoolsports
16 hours agoOld Dog Bites Back | Surviving Barstool S4 Ep. 9
137K4 -
 5:13:04
5:13:04
Right Side Broadcasting Network
7 days agoLIVE REPLAY: TPUSA's America Fest Conference: Day One - 12/19/24
188K28 -
 1:06:01
1:06:01
Man in America
1 day agoPfizer Has Been Caught RED HANDED w/ Dr. Chris Flowers
64.7K50 -
 2:24:15
2:24:15
Slightly Offensive
14 hours ago $19.01 earnedAttempted ASSASSINATION of Nick J Fuentes LEAVES 1 DEAD! | Guest: Mel K & Breanna Morello
54K91