Premium Only Content
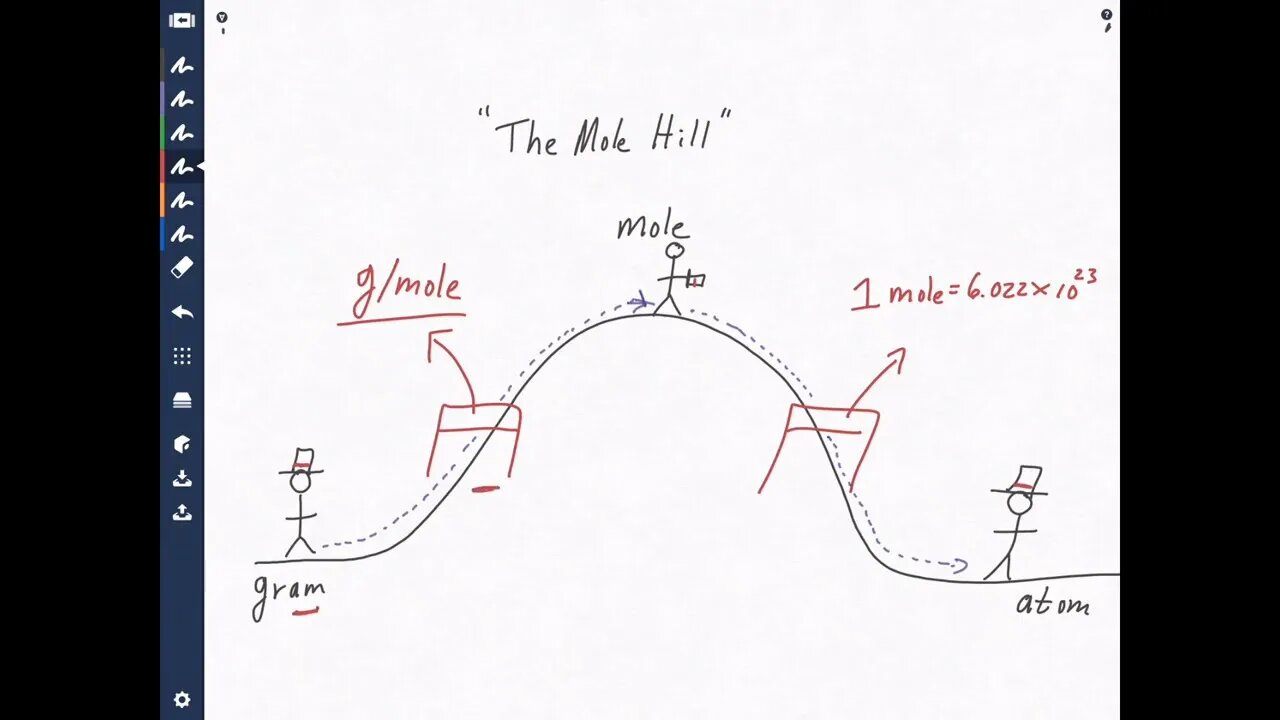
Converting Grams to Moles, Moles to Atoms, Grams to Atoms Avogadro's number
Converting grams to moles, moles to atoms and grams to atoms is fundamental. In fact you will see this on chemistry exams! This video will teach you how to convert grams to moles, how to convert moles to atoms and how to convert grams to atoms. Converting grams to atoms (g to atoms), moles to atoms and grams to moles (g to moles) is easy if you use dimensional analysis and Avogadro's number (6.022 x 10^23). Avogadro's number is often referred to as Avogadro’s Constant. The Avogadro's number definition is 6.022 x 10^23.
Please like this video and leave a comment below! It really helps and is so encouraging.
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
#convertinggramstomoles
#convertmolestograms
#gramstomolesconversion
#convertinggramstoatoms
#convertingmolestoatoms
#convertgtomoles
#avogadroconstant
#avogadrosnumber
#avogadro
#avogadrosnumber
-
 5:58
5:58
Chemistry Tutor
2 years ago $0.02 earnedHow to Draw a Lewis Structure From a Molecular Formula C6H14O2 Help with Chemistry!
141 -
 1:04:23
1:04:23
Benny Johnson
1 hour agoBehind the Scenes With JD Vance on Air Force 2 | VP Gives FLAMETHROWER Speech as Stadium Crowds ROAR
3.41K4 -
 1:36:10
1:36:10
Graham Allen
3 hours agoBiden Admin EXPOSED For Spying On Senators!! + Erika Kirk/JD Vance Take Over Ole Miss!
97.3K40 -
 LIVE
LIVE
LadyDesireeMusic
1 hour agoLive Piano & Convo
239 watching -
 LIVE
LIVE
The Big Mig™
2 hours agoExposed, Current FBI Bosses Behind Operation Arctic Frost
5,124 watching -
 LIVE
LIVE
Badlands Media
4 hours agoBadlands Daily: October 30, 2025
3,944 watching -
 LIVE
LIVE
GrimmHollywood
13 hours ago🔴LIVE • GRIMM HOLLYWOOD • GRIMM RAIDERS • RELEASE DAY •
120 watching -
 LIVE
LIVE
Matt Kohrs
10 hours agoUS China Trade Deal, GDP Report & Mag 7 Earnings || Live Day Trading
385 watching -
 LIVE
LIVE
Wendy Bell Radio
6 hours agoOh SNAP
7,051 watching -
 29:17
29:17
James Klüg
18 hours agoMAGA and Antifa CLASH at Portland ICE Facility
4.71K20