Premium Only Content
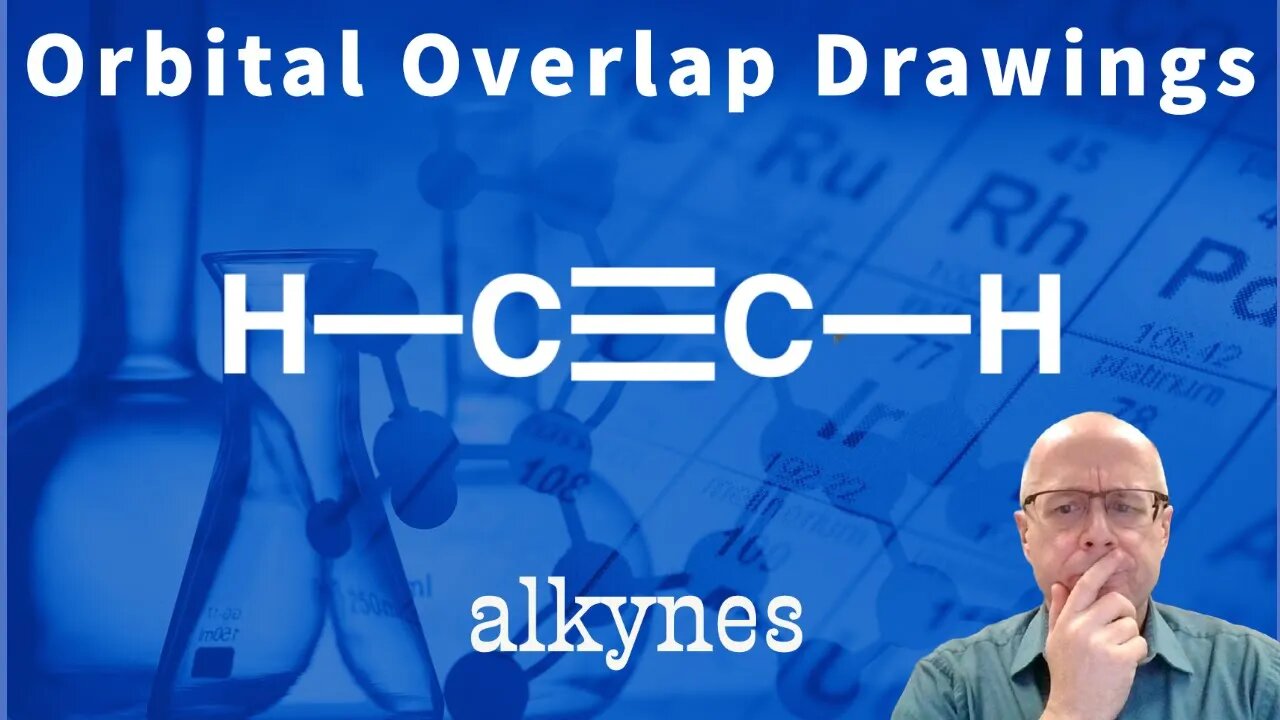
Organic Chemistry Orbital Overlap Problem: Acetylene (triple bond) sp
Being able to visualize the bonding orbitals of an organic molecule is important to your success in organic chemistry. Indeed, understanding orbital bonding and hybrid orbitals will help you to understand reactivity as your organic chemistry class moves forward into more difficult subjects. Sp3, sp2 and sp orbitals are involved in sigma bonding and p orbitals are involved in pii bonding.
In this video I am drawing the orbital overlap drawing of acetylene . In acetylene the carbons are sp hybridized. This means that each carbon has two (2) sp orbitals and two (2) unhybridized p orbitals. The sp orbitals make the sigma bonds and the p orbitals make the pi bonds.
It is important to understand the difference between sigma and pi bonds. It is also important to understand the sigma bond orbitals and the pi bond orbitals.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
https://www.dropbox.com/s/r17auqic2bufklh/alkyne%20hybridized%20orbitals%20.pdf?dl=0
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
#orbitals #sp #acetylene
#organicchemistry
#organiccompounds
#organicproblems
#organictutor
#hybridorbitals
#hybrid
-
 1:09:09
1:09:09
Chemistry Tutor
1 year ago $0.03 earnedAll About the SN1 Mechanism - Lecture 29 has need for more editing
79 -
 14:15
14:15
Tactical Advisor
8 hours agoNew Ruger Glock Clone | Magpul RXM (FIRST LOOK)
12.8K7 -
 7:55
7:55
The Nima Yamini Show
15 hours agoWho Is Controlling Facebook and Instagram? The Truth About George Soros and Meta’s Oversight Board
18.2K26 -
 3:45
3:45
BIG NEM
7 hours agoDiscover Your Ikigai: Finding Your Ultimate Purpose
6.5K1 -
 13:19
13:19
Dermatologist Dr. Dustin Portela
1 day ago $1.71 earnedDo You Have Sebaceous Filaments or Blackheads?
5.15K1 -
 2:21:35
2:21:35
Price of Reason
11 hours agoThe Establishment WORRIES about Elon Musk AGAIN! Superman Trailer Discussion! Sonic 3 Review!
39.6K2 -
 1:14:54
1:14:54
Steve-O's Wild Ride! Podcast
14 hours ago $15.64 earnedZac Brown Reveals The Secrets To HIs Success - Wild Ride #247
56.5K12 -
 4:16:43
4:16:43
JdaDelete
8 hours ago $11.48 earnedProject Zomboid with the Boys | The Great Boner Jam of 2025
35.5K -
 6:00:08
6:00:08
SpartakusLIVE
9 hours agoYoung Spartan STUD teams with old gamers for ultimate BANTER with a SMATTERING of TOXICITY
28.1K -
 1:50:39
1:50:39
Kim Iversen
10 hours agoShocking Proposal: Elon Musk for Speaker of the House?! | IDF Soldiers Reveal Atrocities—'Everyone Is a Terrorist'
73.9K162