Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
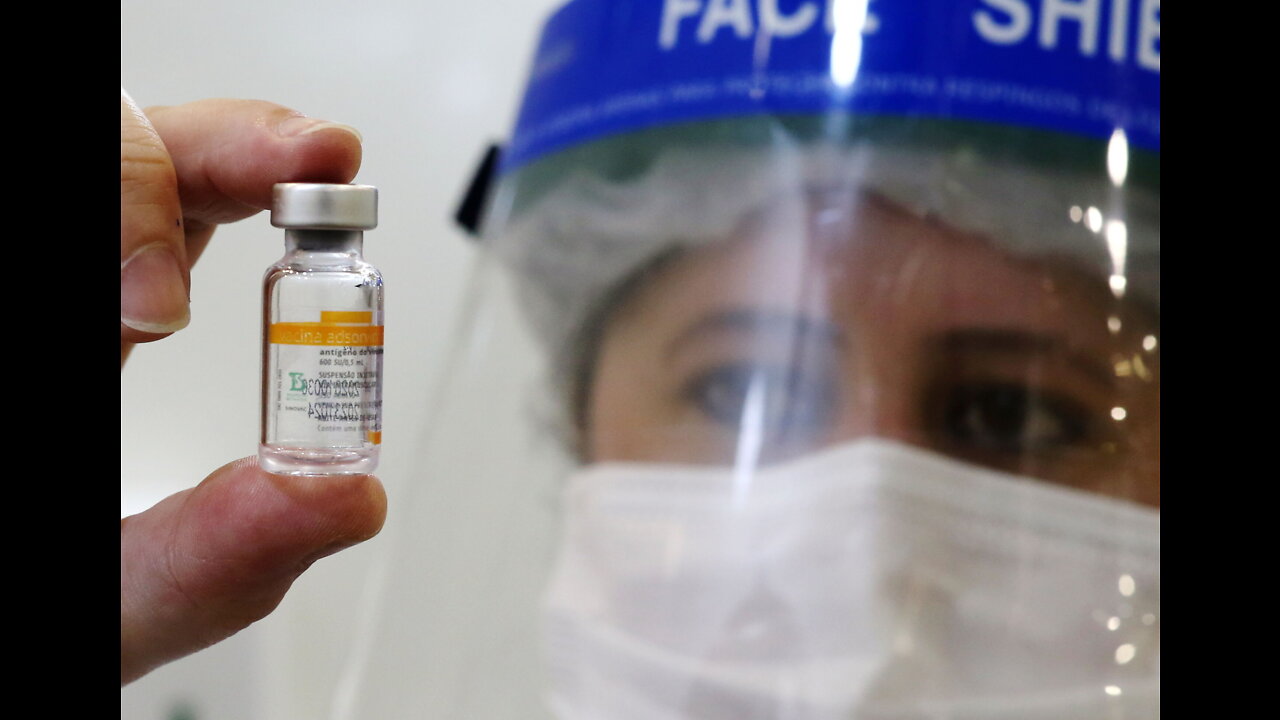
Coronavac approved for emergency use in 18-59 age group in SA
3 years ago
160
The CoronaVac Covid-19 vaccine has been approved for emergency use access by the South African Health Products Regulatory Authority (Sahpra).
The inactivated vaccine — which has already been administered to over 2.5 billion people worldwide — was approved for use in the age group 18-59.
Manufactured by a biopharmaceutical company based in China, Sinovac Biotech, the vaccine will be distributed by their in-country South African partner, the Numolux Group.
Loading 1 comment...
-
 6:58
6:58
IOL-IndependentOnline
27 days ago $0.17 earnedWestern Cape police urged to respond to community demands amid crime surge
9811 -
 LIVE
LIVE
vivafrei
22 hours agoEp. 278: D.C. Peace Wave! Big Tish & Nipple Judge SPANKED! "Maryland Man" Trafficker FREE & MORE?
7,598 watching -
 LIVE
LIVE
SpartakusLIVE
2 hours agoThe BADDEST Duo in WZ Exhibits PEAK Physique || Duos w/ Sophiesnazz to start, quads later
642 watching -
 LIVE
LIVE
Nerdrotic
2 hours ago $0.07 earnedMysteries of 3I/ATLAS | Forbidden Frontier #113
551 watching -
 11:52
11:52
Exploring With Nug
1 hour ago $0.03 earnedWhat’s Hiding Under This Dallas Lake We Found a Vehicle!
7.07K3 -
 2:59:09
2:59:09
Barry Cunningham
8 hours agoBREAKING NEWS: PRESIDENT TRUMP SET TO TAKEOVER CHICAGO AND BOSTON!
26.1K24 -
 LIVE
LIVE
LumpyPotatoX2
2 hours agoSunday Funday on HellDivers - #RumbleGaming
152 watching -
 3:11:52
3:11:52
Esports Awards
5 hours agoEsports Awards: Decade Awards 2025
47.8K3 -
 1:02:58
1:02:58
Sarah Westall
3 hours agoMILITARY WHISTLEBLOWER: How Social Media Military Level Psyops are Manipulating You w/ Patrick Bergy
15.3K4 -
 30:41
30:41
Stephen Gardner
2 hours ago🔥WHITE HOUSE GETS UNEXPECTED BIG WIN!
18.4K15