Premium Only Content
Day 2: FDA Committee meets to discuss the future of Covid vaccines for ages 6 months to 5 years old
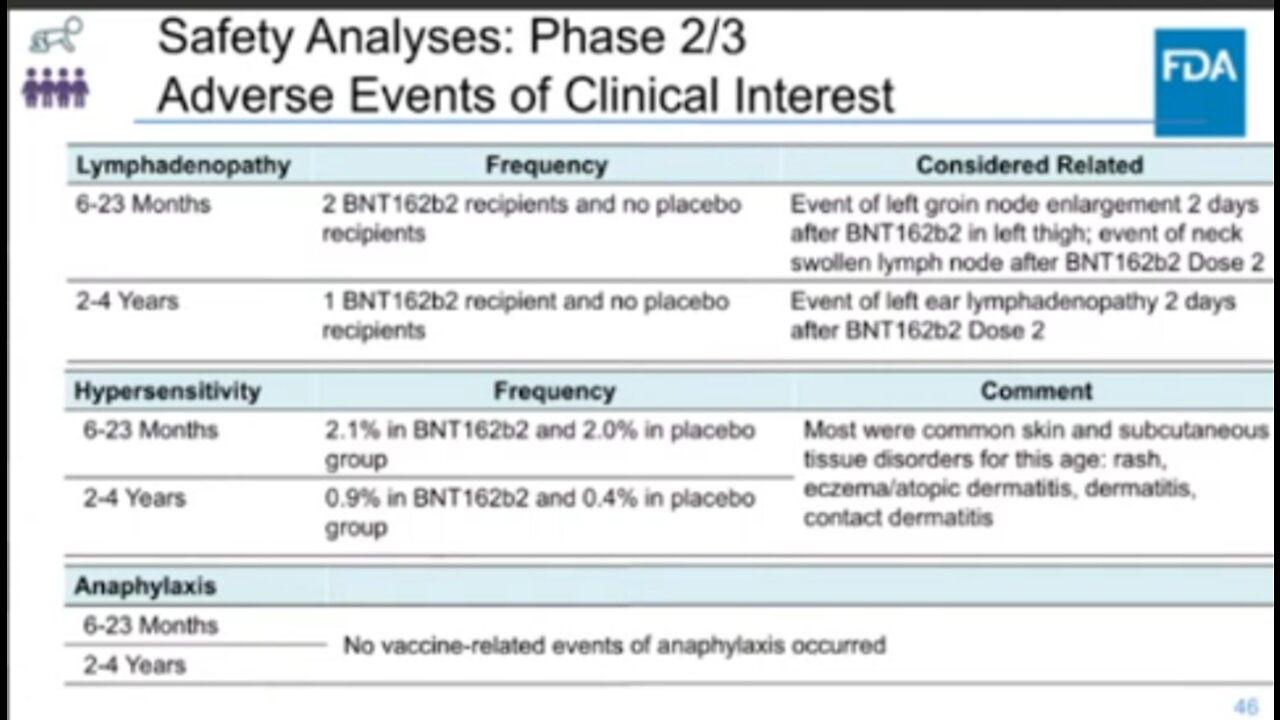
On day 2, June 15,2022, Food and Drug Administration for an upcoming meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss the Moderna EUA request for a COVID-19 vaccine for 6 months through 5 years of age and Pfizer-BioNTech EUA request for 6 months through 4 years of age.The advisory committee under Topic II, the committee will meet in open session to discuss amending the EUA of the Moderna COVID-19 mRNA vaccine to include the administration of the primary series to infants and children 6 months through 4 years of age, and also to discuss amending the EUA of the Pfizer-BioNTech COVID-19 mRNA vaccine to include the administration of the primary series to infants and children 6 months through 4 years of age.
Day 1 meeting:
https://rumble.com/v18rjgs-fda-committee-meets-to-discuss-the-future-of-covid-vaccines-for-ages-6-mont.html
*Vaccines and Related Biological Products Advisory Committee June 14, 2022 Meeting Draft Roster
https://www.fda.gov/media/159155/download
Join Banned Deplorables on Telegram
https://t.me/Banned_Deplorables
-
 2:00:00
2:00:00
Banned Deplorables
10 months agoThe Greatest Show on Earth part 2- Follow the yellow brick road
966 -
 2:36:28
2:36:28
I_Came_With_Fire_Podcast
12 hours agoObama's Treason, Trade War: Season Infinity, and Hunter's Pipe Dream
13.3K -
 2:52:51
2:52:51
TimcastIRL
5 hours agoObama Referred To DOJ For TREASON, Criminal Investigation, CIVIL WAR!! | Timcast IRL
217K114 -
 9:43:32
9:43:32
RalliedLIVE
12 hours ago $5.56 earned10 WINS WITH THE SHOTTY BOYS
100K4 -
 1:35:43
1:35:43
Badlands Media
16 hours agoAltered State S3 Ep. 38: Biolabs, Bitcoin & the Epstein Data Vault
52.5K10 -
 LIVE
LIVE
SpartakusLIVE
4 hours agoLAST Stream Until NEXT WEEK || Going to Florida to hang out w/ a SPECIAL Gaming Buddy
277 watching -
 10:19
10:19
MattMorseTV
1 day ago $11.34 earnedTrump just went SCORCHED EARTH.
70.6K63 -
 1:36:49
1:36:49
RiftTV
7 hours agoHow Much is the Government SPYING on You and STEALING Your DATA?! | Almost Serious | Guest: Matt Kim
34.2K4 -
 1:31:26
1:31:26
Glenn Greenwald
8 hours agoAaron Maté on More Russiagate Fallout, Protests in Ukraine, and Israel's Strikes on Syria With Special Guests John Solomon, Marta Havryshko, and Joshua Landis | SYSTEM UPDATE #491
118K39 -
 1:55:43
1:55:43
Melonie Mac
7 hours agoGo Boom Live Ep 56!
35.5K6